In patients with established chronic coronary syndrome (CCS), the significance of persistent angina is controversial. We aimed to evaluate the prognostic role of persistent angina in symptomatic CCS patients with abnormal stress cardiovascular magnetic resonance (CMR) and altered angiographic findings undergoing percutaneous revascularization.
MethodsWe analyzed 334 CCS patients with Canadian Cardiovascular Society angina class ≥2, perfusion deficits on stress CMR and severe lesions in angiography who underwent medical therapy optimization plus CMR-guided percutaneous revascularization. We investigated the association of persistent angina at 6 months postintervention with subsequent cardiac death, myocardial infarction, and hospital admission.
ResultsAll patients had angina class ≥2 (mean: 2.8±0.7), abnormal stress CMR (mean ischemic burden: 5.8±2.7 segments), and severe angiographic lesions. The angina resolution rates were 81% at 6 months, and 81%, 81%, and 77% at 1, 2, and 5 years, respectively. During a median follow-up of 8.9 years, persistent angina was independently associated with higher rates of subsequent cardiac death (13% vs 4%; HR, 3.7; 95%CI, 1.5-9.2; P=.005), myocardial infarction (24% vs 6%; HR, 4.9; 95%CI, 2.4–9.9; P<.001), and hospital admission for heart failure (27% vs 13%; HR, 2.7; 95%CI, 1.5–5.2; P=.001).
ConclusionsIn CCS patients with robust diagnostic evidence from symptoms, stress CMR, and angiography, persistent angina after percutaneous revascularization is a strong predictor of subsequent cardiac death, myocardial infarction, and hospital admission for heart failure.
Keywords
Angina, also known as angina pectoris, is the pivotal symptom and a universally recognized indicator of myocardial ischemia. In patients with established chronic coronary syndrome (CCS), the significance of myocardial ischemia is controversial.1
One of the main goals of physicians is to alleviate symptoms, which requires comprehensive, personalized management, including optimized medical therapy for CCS patients.2 Randomized trials have repeatedly failed to demonstrate that the widespread use of percutaneous coronary intervention (PCI) provides substantial therapeutic benefits in terms of reducing mortality or myocardial infarction rates,3 and its effect on angina resolution has also been questioned.4 Nonetheless, ischemia-guided coronary revascularization remains a cornerstone for improving angina symptoms in patients with significant ischemic burden.1,2,5
The prevalence of persistent angina within the first year after treatment intervention has varied widely in previous studies6 and its association with subsequent hard clinical events remains unclear.7,8 This may be partly due to significant disparities between study groups. Assessment of this issue is needed in a homogeneous cohort using strict clinical, cardiac imaging, and angiographic inclusion criteria.9 In this context, vasodilator stress cardiovascular magnetic resonance (CMR) is the first-choice tool for diagnosis, risk stratification, and management guidance.2,10,11
In a registry of CCS patients with limiting angina symptoms, significant perfusion deficits on stress CMR and severe coronary lesions on angiography, we investigated the associations between postintervention (comprising medical therapy plus CMR-guided PCI) persistent angina and the occurrence of subsequent cardiac death, myocardial infarction, and admission for heart failure.
METHODSRegistryThis project originates from a large registry of 6700 consecutive patients who underwent vasodilator stress CMR for known or suspected CCS in our health department from 2001 to 2016.10 For the specific purpose of this study, we retrospectively selected 334 patients with limiting angina symptoms (angina class ≥2), evidence of myocardial ischemia on stress perfusion CMR, and severe lesions on coronary angiography. All patients were evaluated in specialized outpatient clinics and underwent treatment intervention, which included medical therapy optimization plus CMR-guided PCI.
The objective of the present study was to analyze the effect of persistent angina (at 6 months after treatment intervention) on subsequent cardiac events. Patients who experienced cardiac death, myocardial infarction, or any cardiovascular admission within the first 6 months after inclusion were censored from the study. The flow chart and reasons for exclusion are shown in . The attending cardiologists had full and unrestricted access to all variables presented in this study and decision-making was left to their discretion.
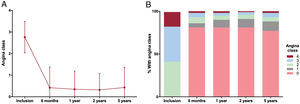
Canadian Cardiovascular Society angina class (range 0-4, with class 0 indicating no angina and class 4 indicating angina at rest) was recorded at patient inclusion and at 6 months, 1 year, 2 years, and 5 years thereafter (). Persistent angina was defined as angina class ≥1 at 6 months postintervention.
Our registry was conducted in accordance with the Declaration of Helsinki. Events were collected retrospectively. In September 2018, the local ethics committee approved the study and the retrospective review of events occurring in the patients included in the registry, exempting the need for informed consent. Authorized personnel conducted this review using the electronic regional health system registry from October to November 2018.
CMR data analysisTechnical aspects related to the CMR studies are detailed in the supplementary data and described elsewhere.10,11
Left ventricular (LV) ejection fraction (%) and LV end-diastolic and end-systolic volumes indices (mL/m2) were quantified using cine images. Based on the 17-segment model,12 2 segmental postcontrast CMR indices were visually assessed:
- 1.
Ischemic burden: The ischemic burden was defined by the number of ischemic segments (those showing perfusion defects on poststress imaging). Only patients with >1 ischemic segment were included in the study group.
- 2.
Late gadolinium enhancement extent: This was visually defined as the number of segments showing l late gadolinium enhancement.
CMR-related cardiac catheterization and percutaneous coronary intervention (PCI) were defined as procedures performed within 3 months after the index vasodilator stress CMR study, provided that patients were not hospitalized for cardiovascular causes during that period (in which case, they were censored). This definition has been previously used by our group10 and by other authors.13
Affected vessels were defined as those with >2mm diameter and at least 1 stenosis >70%. The presence of left main or multivessel disease, chronic total occlusion, stenosis >90%, proximal left anterior descending artery disease, and the Bypass Angioplasty Revascularization Investigation (BARI) score were also recorded. Incomplete revascularization was defined as the presence of >1 ischemic segment based on affected vessels not treated with PCI.
EndpointIn a cohort of symptomatic CCS patients with robust evidence of myocardial ischemia, indicated by perfusion deficits on stress CMR and altered findings on coronary angiography, the endpoint was the association between persistent angina at 6 months after treatment intervention (including medical therapy and CMR-guided PCI) and the subsequent occurrence of cardiac death, myocardial infarction, and admission for heart failure.
Statistical analysisData were tested for normal distribution using the Kolmogorov-Smirnov test. Continuous normally distributed data were expressed as the mean±standard deviation and compared using the unpaired Student t-test. Non-parametric data are expressed as the median with the interquartile range and were compared using the Mann-Whitney U test. Group percentages were compared using the chi-square test or the Fisher exact test, as appropriate. P values for trends were used to compare more than 2 percentages.
Forward stepwise multiple binary logistic regression, after adjustment for variables with a 2-tailed P value <.05 in univariate analyses, was used to predict the occurrence of persistent angina (defined as angina class ≥1 at 6 months postintervention). Odds ratios (OR) with the respective 95% confidence intervals (95%CI) were calculated.
Incidence rates of cardiac death, myocardial infarction, and admission for heart failure (expressed as events per 100 person-years) were determined. Associations between persistent angina and the cumulative incidence of cardiac events were assessed using the Pepe and Mori test. The adjusted effects of persistent angina on the occurrence of cardiac death, myocardial infarction and admission for heart failure were determined using Fine-Gray hazard models for competing events. This approach allows adjustment, if appropriate, for the risk inherent in prior events.14 The number of competing events and the time elapsed (in weeks) between them are shown in . The respective adjusted survival curves were obtained. Variables with a P value <.05 in univariate analyses were used for adjustments. Hazard ratios (HR) with the respective 95%CI were computed. The proportional hazards assumption, based on Schoenfeld residuals, was satisfied with a P value >.05.
To mitigate the potential impact of the long inclusion period on the results, multivariate analyses were adjusted for the calendar year of inclusion. Additionally, the dynamics of angina class and the associations of persistent angina with the occurrence of cardiac events were assessed separately in patients enrolled within the last 5 years of the inclusion period.
The collinearity of variables tested in multivariate models was assessed using the tolerance statistic (excessive if <0.20) and the variance inflation factor (excessive if >5).
Statistical significance was set at a 2-tailed P value <.05. The SPSS statistical package (version 15.0, SPSS Inc, Chicago, Illinois) and STATA (version 9.0, StataCorp, College Station, Texas) were used throughout.
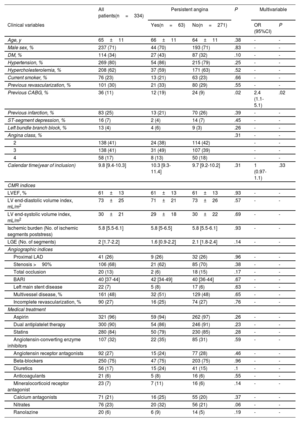
RESULTSThe median follow-up period was 8.9 years (463 weeks [range, 236–690 weeks]). The mean age was 65±11 years, and 71% of the patients were male. All patients in the study group had angina class ≥2, abnormal stress CMR (mean ischemic burden 5.8±2.7 segments), and one or more severe angiographic lesions (56% with multivessel disease). The characteristics of the entire study group are displayed in table 1.
Baseline characteristics of the whole group. Univariable and multivariable associations with the occurrence of persistent angina
| All patients(n=334) | Persistent angina | P | Multivariable | |||
|---|---|---|---|---|---|---|
| Clinical variables | Yes(n=63) | No(n=271) | OR (95%CI) | P | ||
| Age, y | 65±11 | 66±11 | 64±11 | .38 | - | - |
| Male sex, % | 237 (71) | 44 (70) | 193 (71) | .83 | - | - |
| DM, % | 114 (34) | 27 (43) | 87 (32) | .10 | - | - |
| Hypertension, % | 269 (80) | 54 (86) | 215 (79) | .25 | - | - |
| Hypercholesterolemia, % | 208 (62) | 37 (59) | 171 (63) | .52 | - | - |
| Current smoker, % | 76 (23) | 13 (21) | 63 (23) | .66 | - | - |
| Previous revascularization, % | 101 (30) | 21 (33) | 80 (29) | .55 | - | - |
| Previous CABG, % | 36 (11) | 12 (19) | 24 (9) | .02 | 2.4 (1.1-5.1) | .02 |
| Previous infarction, % | 83 (25) | 13 (21) | 70 (26) | .39 | - | - |
| ST-segment depression, % | 16 (7) | 2 (4) | 14 (7) | .45 | - | - |
| Left bundle branch block, % | 13 (4) | 4 (6) | 9 (3) | .26 | - | - |
| Angina class, % | .31 | - | - | |||
| 2 | 138 (41) | 24 (38) | 114 (42) | - | - | |
| 3 | 138 (41) | 31 (49) | 107 (39) | - | - | |
| 4 | 58 (17) | 8 (13) | 50 (18) | - | - | |
| Calendar time(year of inclusion) | 9.8 [9.4-10.3] | 10.3 [9.3-11.4] | 9.7 [9.2-10.2] | .31 | 1 (0.97-1.1) | .33 |
| CMR indices | ||||||
| LVEF, % | 61±13 | 61±13 | 61±13 | .93 | - | - |
| LV end-diastolic volume index, mL/m2 | 73±25 | 71±21 | 73±26 | .57 | - | - |
| LV end-systolic volume index, mL/m2 | 30±21 | 29±18 | 30±22 | .69 | - | - |
| Ischemic burden (No. of ischemic segments poststress) | 5.8 [5.5-6.1] | 5.8 [5-6.5] | 5.8 [5.5-6.1] | .93 | - | - |
| LGE (No. of segments) | 2 [1.7-2.2] | 1.6 [0.9-2.2] | 2.1 [1.8-2.4] | .14 | - | - |
| Angiographic indices | ||||||
| Proximal LAD | 41 (26) | 9 (26) | 32 (26) | .96 | - | - |
| Stenosis >90% | 106 (68) | 21 (62) | 85 (70) | .38 | - | - |
| Total occlusion | 20 (13) | 2 (6) | 18 (15) | .17 | - | - |
| BARI | 40 [37-44] | 42 [34-49] | 40 [36-44] | .67 | - | - |
| Left main stent disease | 22 (7) | 5 (8) | 17 (6) | .63 | - | - |
| Multivessel disease, % | 161 (48) | 32 (51) | 129 (48) | .65 | - | - |
| Incomplete revascularization, % | 90 (27) | 16 (25) | 74 (27) | .76 | - | - |
| Medical treatment | ||||||
| Aspirin | 321 (96) | 59 (94) | 262 (97) | .26 | - | - |
| Dual antiplatelet therapy | 300 (90) | 54 (86) | 246 (91) | .23 | - | - |
| Statins | 280 (84) | 50 (79) | 230 (85) | .28 | - | - |
| Angiotensin-converting enzyme inhibitors | 107 (32) | 22 (35) | 85 (31) | .59 | - | - |
| Angiotensin receptor antagonists | 92 (27) | 15 (24) | 77 (28) | .46 | - | - |
| Beta-blockers | 250 (75) | 47 (75) | 203 (75) | .96 | - | - |
| Diuretics | 56 (17) | 15 (24) | 41 (15) | .1 | - | - |
| Anticoagulants | 21 (6) | 5 (8) | 16 (6) | .55 | - | - |
| Mineralocorticoid receptor antagonist | 23 (7) | 7 (11) | 16 (6) | .14 | - | - |
| Calcium antagonists | 71 (21) | 16 (25) | 55 (20) | .37 | - | - |
| Nitrates | 76 (23) | 20 (32) | 56 (21) | .06 | - | - |
| Ranolazine | 20 (6) | 6 (9) | 14 (5) | .19 | - | - |
95%CI, 95% confidence interval; BARI, Bypass Angioplasty Revascularization Investigation; CMR, cardiovascular magnetic resonance; DM, diabetes mellitus; CABG, coronary artery bypass grafting; LAD, left anterior descending artery; LGE, late gadolinium enhancement; LV, left ventricular; LVEF, left ventricular ejection fraction; OR, odds ratio.
The data are expressed as No. (%) or mean±standard deviation, or median [interquartile range].
Mean angina class upon inclusion was 2.8±0.7. A significant and steady decrease in angina class occurred throughout the months and years after the intervention (figure 1). Persistent angina at 6 months was detected in 63 patients (19%). The rate of angina-free patients (CCS class 0) increased dramatically at 6 months, and this trend persisted at subsequent time points (figure 1). The same trend was observed in the 145 patients enrolled within the last 5 years of the inclusion period () as well as in separate analyses performed for male and female patients ().
The characteristics and medical treatment of patients with and without persistent angina are shown in table 1. The most frequent cause of persistent angina noted by the attending cardiologists was incomplete coronary revascularization (n=21, 33%). Pre-existing diffuse coronary atherosclerosis (n=10, 16%), in-stent restenosis (n=6, 9%), coronary artery disease progression (n=5, 8%), and coronary vasospasm (n=1, 2%) were also associated with persistent angina. In 20 cases (32%), the cause of persistent angina was unclear. No gender differences were observed regarding the occurrence of persistent angina (table 1).
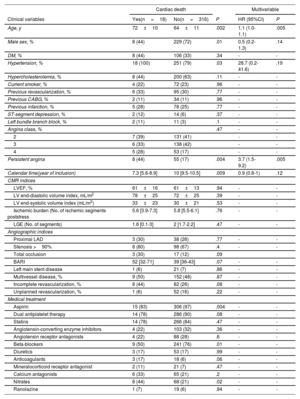
Association of persistent angina with cardiac deathCardiac death occurred in 18 patients (5%), representing 0.6 cardiac deaths per 100 person-years. Patient characteristics related to cardiac death are shown in table 2.
Univariable and multivariable associations with the occurrence of cardiac death
| Cardiac death | Multivariable | ||||
|---|---|---|---|---|---|
| Clinical variables | Yes(n=18) | No(n=316) | P | HR (95%CI) | P |
| Age, y | 72±10 | 64±11 | .002 | 1.1 (1.0-1.1) | .005 |
| Male sex, % | 8 (44) | 229 (72) | .01 | 0.5 (0.2-1.3) | .14 |
| DM, % | 8 (44) | 106 (33) | .34 | - | - |
| Hypertension, % | 18 (100) | 251 (79) | .03 | 28.7 (0.2-41.6) | .19 |
| Hypercholesterolemia, % | 8 (44) | 200 (63) | .11 | - | - |
| Current smoker, % | 4 (22) | 72 (23) | .96 | - | - |
| Previous revascularization, % | 6 (33) | 95 (30) | .77 | - | - |
| Previous CABG, % | 2 (11) | 34 (11) | .96 | - | - |
| Previous infarction, % | 5 (28) | 78 (25) | .77 | - | - |
| ST-segment depression, % | 2 (12) | 14 (6) | .37 | - | - |
| Left bundle branch block, % | 2 (11) | 11 (3) | .1 | - | - |
| Angina class, % | .47 | - | - | ||
| 2 | 7 (39) | 131 (41) | - | - | |
| 3 | 6 (33) | 138 (42) | - | - | |
| 4 | 5 (28) | 53 (17) | - | - | |
| Persistent angina | 8 (44) | 55 (17) | .004 | 3.7 (1.5-9.2) | .005 |
| Calendar time(year of inclusion) | 7.3 [5.6-8.9] | 10 [9.5-10.5] | .009 | 0.9 (0.8-1) | .12 |
| CMR indices | |||||
| LVEF, % | 61±16 | 61±13 | .94 | - | - |
| LV end-diastolic volume index, mL/m2 | 78±25 | 72±25 | .39 | - | - |
| LV end-systolic volume index (mL/m2) | 33±23 | 30±21 | .53 | - | - |
| Ischemic burden (No. of ischemic segments poststress | 5.6 [3.9-7.3] | 5.8 [5.5-6.1] | .76 | - | - |
| LGE (No. of segments) | 1.6 [0.1-3] | 2 [1.7-2.2] | .47 | - | - |
| Angiographic indices | |||||
| Proximal LAD | 3 (30) | 38 (26) | .77 | - | - |
| Stenosis >90% | 8 (80) | 98 (67) | .4 | - | - |
| Total occlusion | 3 (30) | 17 (12) | .09 | ||
| BARI | 52 [32-71] | 39 [36-43] | .07 | - | - |
| Left main stent disease | 1 (6) | 21 (7) | .86 | - | - |
| Multivessel disease, % | 9 (50) | 152 (48) | .87 | - | - |
| Incomplete revascularization, % | 8 (44) | 82 (26) | .08 | - | - |
| Unplanned revascularization, % | 1 (6) | 52 (16) | .22 | - | - |
| Medical treatment | |||||
| Aspirin | 15 (83) | 306 (97) | .004 | - | - |
| Dual antiplatelet therapy | 14 (78) | 286 (90) | .08 | - | - |
| Statins | 14 (78) | 266 (84) | .47 | - | - |
| Angiotensin-converting enzyme inhibitors | 4 (22) | 103 (32) | .36 | - | - |
| Angiotensin receptor antagonists | 4 (22) | 88 (28) | .6 | - | - |
| Beta-blockers | 9 (50) | 241 (76) | .01 | - | - |
| Diuretics | 3 (17) | 53 (17) | .99 | - | - |
| Anticoagulants | 3 (17) | 18 (6) | .06 | - | - |
| Mineralocorticoid receptor antagonist | 2 (11) | 21 (7) | .47 | - | - |
| Calcium antagonists | 6 (33) | 65 (21) | .2 | - | - |
| Nitrates | 8 (44) | 68 (21) | .02 | - | - |
| Ranolazine | 1 (7) | 19 (6) | .94 | - | - |
95%CI, 95% confidence interval; BARI, Bypass Angioplasty Revascularization Investigation; CMR, cardiovascular magnetic resonance; DM, diabetes mellitus; CABG, coronary artery bypass grafting; HR, hazard ratio; LAD, left anterior descending artery; LGE, late gadolinium enhancement; LV, left ventricular; LVEF, left ventricular ejection fraction.
The data are expressed as No. (%) or mean±standard deviation, or median [interquartile range].
Cardiac death was associated with a higher angina class at 6 months, 1 year, and 2 years, but not at inclusion. Similarly, the percentage of patients with angina class ≥1 was higher at 6 months, 1 year, and 2 years postintervention in those who subsequently experienced cardiac death compared with survivors ().
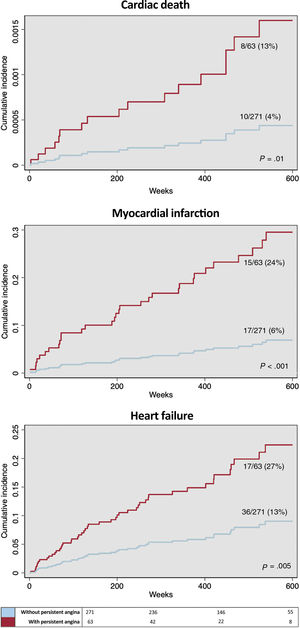
Cardiac death was more prevalent in patients with persistent angina (angina class ≥1 at 6 months postintervention): 8/63 (13%) vs 10/271 (4%); 1.6 vs 0.5 cardiac deaths per 100 person-years (P=.002). After adjustment for variables significantly associated with cardiac death (table 2), persistent angina emerged as an independent predictor (HR, 3.7; 95%CI, 1.5-9.2; P=.005, figure 2).
Association of persistent angina with cardiac events. Adjusted survival curves. Persistent angina (angina class ≥1 at 6 months after intervention) was associated with a higher rate of subsequent cardiac events during follow-up (A: cardiac death; B: myocardial infarction; C: heart failure). Survival curves are adjusted for competing events and cofactors independently associated with the occurrence of the respective cardiac events in multivariable analyses (table 2, table 3, and table 4).
Myocardial infarction was detected in 32 patients (10%), representing 1.1 myocardial infarctions per 100 person-years. Patient characteristics associated with the occurrence of myocardial infarction are shown in table 3.
Univariable and multivariable associations with the occurrence of myocardial infarction
| Myocardial infarction | Multivariable | ||||
|---|---|---|---|---|---|
| Clinical variables | Yes(n=32) | No(n=302) | P | HR (95%CI) | P |
| Age, y | 67±11 | 64±11 | .16 | - | - |
| Male sex, % | 17 (53) | 220 (73) | .02 | 0.4 (0.2-0.8) | .01 |
| DM, % | 14 (44) | 100 (33) | .23 | - | - |
| Hypertension, % | 29 (91) | 240 (79) | .13 | - | - |
| Hypercholesterolemia, % | 20 (62) | 188 (62) | .98 | - | - |
| Current smoker, % | 8 (25) | 68 (22) | .75 | - | - |
| Previous revascularization, % | 10 (31) | 91 (30) | .9 | - | - |
| Previous CABG, % | 4 (12) | 32 (11) | .74 | - | - |
| Previous infarction, % | 8 (25) | 75 (25) | .98 | - | - |
| ST-segment depression, % | 3 (11) | 13 (6) | .34 | - | - |
| Left bundle branch block, % | 1 (3) | 12 (4) | .81 | - | - |
| Angina class, % | .8 | - | - | ||
| 2 | 15 (47) | 123 (41) | - | - | |
| 3 | 12 (37) | 126 (42) | - | - | |
| 4 | 5 (16) | 53 (17) | - | - | |
| Persistent angina | 15 (47) | 48 (16) | <.001 | 4.9 (2.4-9.9) | <.001 |
| Calendar time(year of inclusion) | 8.4 [6.8-10] | 10 [9.5-10.5] | .05 | 0.9 (0.9-1.0) | .29 |
| CMR indices | |||||
| LVEF, % | 63±13 | 61±13 | .53 | - | - |
| LV end-diastolic volume index, mL/m2 | 68±19 | 73±26 | .31 | - | -, |
| LV end-systolic volume index, mL/m2 | 27±17 | 31±21 | .36 | - | - |
| Ischemic burden (No. of ischemic segments poststress | 5.5 [4.4-6.6] | 5.8 [5.5-6.1] | .51 | - | - |
| LGE (No. of segments) | 1.6 [0.8-2.4] | 2 [1.7-2.3] | .37 | - | - |
| Angiographic indices | |||||
| Proximal LAD | 4 (21) | 37 (27) | .59 | - | - |
| Stenosis >90% | 10 (53) | 96 (70) | .13 | - | - |
| Total occlusion | 2 (10) | 18 (13) | .75 | - | - |
| BARI | 42 [33-51] | 40 [36-44] | .69 | - | - |
| Left main stent disease | 1 (3) | 21 (7) | .41 | - | - |
| Multivessel disease, % | 14 (44) | 147 (49) | .6 | - | - |
| Incomplete revascularization, % | 8 (25) | 82 (27) | .79 | - | - |
| Unplanned revascularization, % | 8 (25) | 45 (15) | .14 | - | - |
| Medical treatment | |||||
| Aspirin | 30 (94) | 291 (96) | .47 | - | - |
| Dual antiplatelet therapy | 30 (94) | 270 (89) | .44 | - | - |
| Statins | 24 (75) | 256 (85) | .15 | - | - |
| Angiotensin-converting enzyme inhibitors | 9 (28) | 98 (32) | .62 | - | - |
| Angiotensin receptor antagonists | 8 (25) | 84 (28) | .73 | - | - |
| Beta-blockers | 22 (69) | 228 (75) | .4 | - | - |
| Diuretics | 10 (31) | 46 (15) | .02 | - | - |
| Anticoagulants | 3 (9) | 18 (6) | .45 | - | - |
| Mineralocorticoid receptor antagonist | 4 (12) | 19 (6) | .19 | - | - |
| Calcium antagonists | 7 (22) | 64 (21) | .93 | - | - |
| Nitrates | 10 (31) | 66 (22) | .23 | - | - |
| Ranolazine | 3 (9) | 17 (6) | .4 | - | - |
95%CI, 95% confidence interval; BARI, Bypass Angioplasty Revascularization Investigation; CMR, cardiovascular magnetic resonance; DM, diabetes mellitus; CABG, coronary artery bypass grafting; HR, hazard ratio; LAD, left anterior descending artery; LGE, late gadolinium enhancement; LV, left ventricular; LVEF, left ventricular ejection fraction.
The data are expressed as No. (%) or mean±standard deviation, or median [interquartile range].
Patients with incident myocardial infarction exhibited higher angina class and a greater rate of angina class ≥1 at 6 months, and at 1 year, 2 years, and 5 years, but not at inclusion, compared with those who were free of myocardial infarction during follow-up ().
Myocardial infarction occurred more frequently in patients with persistent angina: 15/63 (24%) vs 17/271 (6%): 2.7 vs 0.7 myocardial infarctions per 100 person-years (P<.001). After adjustment for myocardial infarction-related variables (table 3), persistent angina emerged as an independent risk predictor (HR, 4.9; 95%CI, 2.4–9.9, P<.001, figure 2).
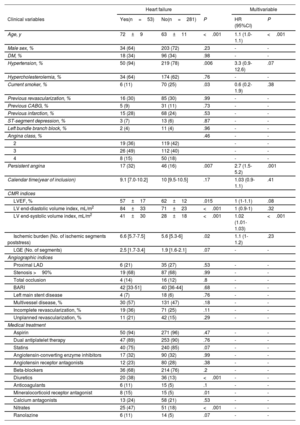
Association of persistent angina with admission for heart failureIncident admission for heart failure was registered in 53 patients (16%), representing 1.9 admissions for heart failure per 100 person-years. Variables associated with heart failure are shown in table 4.
Univariable and multivariable associations with the occurrence of heart failure
| Heart failure | Multivariable | ||||
|---|---|---|---|---|---|
| Clinical variables | Yes(n=53) | No(n=281) | P | HR (95%CI) | P |
| Age, y | 72±9 | 63±11 | <.001 | 1.1 (1.0-1.1) | <.001 |
| Male sex, % | 34 (64) | 203 (72) | .23 | - | - |
| DM, % | 18 (34) | 96 (34) | .98 | - | - |
| Hypertension, % | 50 (94) | 219 (78) | .006 | 3.3 (0.9-12.6) | .07 |
| Hypercholesterolemia, % | 34 (64) | 174 (62) | .76 | - | - |
| Current smoker, % | 6 (11) | 70 (25) | .03 | 0.6 (0.2-1.9) | .38 |
| Previous revascularization, % | 16 (30) | 85 (30) | .99 | - | - |
| Previous CABG, % | 5 (9) | 31 (11) | .73 | - | - |
| Previous infarction, % | 15 (28) | 68 (24) | .53 | - | - |
| ST-segment depression, % | 3 (7) | 13 (6) | .87 | - | - |
| Left bundle branch block, % | 2 (4) | 11 (4) | .96 | - | - |
| Angina class, % | .46 | - | - | ||
| 2 | 19 (36) | 119 (42) | - | - | |
| 3 | 26 (49) | 112 (40) | - | - | |
| 4 | 8 (15) | 50 (18) | - | - | |
| Persistent angina | 17 (32) | 46 (16) | .007 | 2.7 (1.5-5.2) | .001 |
| Calendar time(year of inclusion) | 9.1 [7.0-10.2] | 10 [9.5-10.5] | .17 | 1.03 (0.9-1.1) | .41 |
| CMR indices | |||||
| LVEF, % | 57±17 | 62±12 | .015 | 1 (1-1.1) | .08 |
| LV end-diastolic volume index, mL/m2 | 84±33 | 71±23 | <.001 | 1 (0.9-1) | .32 |
| LV end-systolic volume index, mL/m2 | 41±30 | 28±18 | <.001 | 1.02 (1.01-1.03) | <.001 |
| Ischemic burden (No. of ischemic segments poststress) | 6.6 [5.7-7.5] | 5.6 [5.3-6] | .02 | 1.1 (1-1.2) | .23 |
| LGE (No. of segments) | 2.5 [1.7-3.4] | 1.9 [1.6-2.1] | .07 | - | - |
| Angiographic indices | |||||
| Proximal LAD | 6 (21) | 35 (27) | .53 | - | - |
| Stenosis >90% | 19 (68) | 87 (68) | .99 | - | - |
| Total occlusion | 4 (14) | 16 (12) | .8 | - | - |
| BARI | 42 [33-51] | 40 [36-44] | .68 | - | - |
| Left main stent disease | 4 (7) | 18 (6) | .76 | - | - |
| Multivessel disease, % | 30 (57) | 131 (47) | .18 | - | - |
| Incomplete revascularization, % | 19 (36) | 71 (25) | .11 | - | - |
| Unplanned revascularization, % | 11 (21) | 42 (15) | .29 | - | - |
| Medical treatment | |||||
| Aspirin | 50 (94) | 271 (96) | .47 | - | - |
| Dual antiplatelet therapy | 47 (89) | 253 (90) | .76 | - | - |
| Statins | 40 (75) | 240 (85) | .07 | - | - |
| Angiotensin-converting enzyme inhibitors | 17 (32) | 90 (32) | .99 | - | - |
| Angiotensin receptor antagonists | 12 (23) | 80 (28) | .38 | - | - |
| Beta-blockers | 36 (68) | 214 (76) | .2 | - | - |
| Diuretics | 20 (38) | 36 (13) | <.001 | - | - |
| Anticoagulants | 6 (11) | 15 (5) | .1 | - | - |
| Mineralocorticoid receptor antagonist | 8 (15) | 15 (5) | .01 | - | - |
| Calcium antagonists | 13 (24) | 58 (21) | .53 | - | - |
| Nitrates | 25 (47) | 51 (18) | <.001 | - | - |
| Ranolazine | 6 (11) | 14 (5) | .07 | - | - |
95%CI, 95% confidence interval; BARI, Bypass Angioplasty Revascularization Investigation; CMR, cardiovascular magnetic resonance; DM, diabetes mellitus; CABG, coronary artery bypass grafting; HR, hazard ratio; LAD, left anterior descending artery; LGE, late gadolinium enhancement; LV, left ventricular; LVEF, left ventricular ejection fraction.
The data are expressed as No. (%) or mean±standard deviation, or median [interquartile range].
Incident admission for heart failure correlated with a higher angina class and a larger percentage of patients with angina class ≥1 at 6 months, as well as at 1 and 2 years, but not at inclusion ().
Admission for heart failure was more frequent in patients with persistent angina: 17/63 (27%) vs 36/271 (13%); 3.2 vs 1.5 admissions for heart failure per 100 person-years (P<.001). Once adjusted for variables significantly associated with incident admission for heart failure (table 4), persistent angina emerged as an independent predictor (HR, 2.7; 95%CI, 1.5-5.2, P=.001, figure 2).
The associations of persistent angina with the occurrence of cardiac events persisted in the separate analyses of the 145 patients enrolled within the last 5 years of the inclusion period ().
DISCUSSIONThe main finding of the present study is that in symptomatic CCS patients with limiting angina and evidence of underlying myocardial ischemia based on simultaneous altered stress CMR and severe lesions in angiography, persistent angina after medical treatment optimization plus PCI is infrequent but represents a potent long-term predictor of subsequent cardiac death, myocardial infarction, and readmission for heart failure (figure 3).
Central illustration. In a cohort of 334 CCS patients with robust diagnostic evidence (angina class ≥2, perfusion deficits on stress CMR and severe lesions in angiography), sustained improvement of angina symptoms can be achieved in most patients after treatment intervention (medical therapy plus CMR-guided PCI). Persistent angina (angina class ≥1 at 6 months after intervention) exerts deleterious effects in terms of subsequent cardiac events. CCS, chronic coronary syndrome; CMR, cardiovascular magnetic resonance; PCI, percutaneous coronary intervention.
The incidence of persistent angina after optimal treatment in CCS patients has varied widely in previous studies, with rates of up to 60%.6,15 These fluctuations may be partly attributed to significant disparities among the study groups.4–8 We evaluated a homogeneous series of patients with at least moderately limiting angina symptoms (angina class ≥2) and robust evidence of significant underlying myocardial ischemia (altered stress perfusion CMR and severe disease on coronary angiography). Medical treatment optimization and stress CMR-guided PCI were implemented.
In this scenario, only 19% of patients exhibited persistent angina 6 months after treatment optimization. Furthermore, this improvement was sustained throughout the following months and years, with the rate of angina-free patients being 77% at 5 years.
Two main factors may explain the low incidence of persistent angina in our series compared with previous registries. First, our analysis involved a CCS patient population in whom the physiological and anatomical diagnosis of myocardial ischemia was robustly established, allowing reasonable expectations of treatment success. Previous studies reported persistent chest pain during follow-up often due to reasons unrelated to flow-limiting atherosclerotic plaques (such as atypical symptoms, anxiety, or microvascular angina), which could have reduced treatment effectiveness.6,15 Nevertheless, in the present study, persistent angina was primarily related to incompletely revascularized atherosclerotic burden or pre-existing diffuse coronary disease. Second, we used a personalized strategy in all patients who included stress CMR to localize ischemia and guide revascularization. This approach has been shown to be effective in the management of CCS patients10,11 and may also have contributed to the low rate of persistent angina detected in our series after intervention.
In summary, our results indicate that with the available therapeutic options, angina resolution or sustained amelioration of symptoms can be achieved in the vast majority of CCS patients with limiting angina, significant myocardial ischemia, and severe coronary lesions.
Persistent angina and patient outcomesThe deleterious effects of angina symptoms have been amply demonstrated.16 Furthermore, persistent angina has been consistently associated with worse clinical outcomes in acute coronary syndromes.17 Nevertheless, the significance of persistent (postintervention) angina in CCS remains controversial.7–9 Mentz et al.,18 and Ono et al.7 found an association between persistent angina and more frequent rehospitalizations and repeat revascularizations, but not with long-term mortality.7
In CCS patients with angina symptoms, the demonstration of myocardial ischemia through cardiac imaging confirms the diagnosis and identifies a subset of patients at high risk for subsequent events.10,11,19 However, the prognostic impact of persistent angina after optimization of medical therapy plus CMR-guided PCI in a cohort of CCS patients with severe coronary lesions and perfusion deficits on stress CMR has not yet been explored. In our series, after comprehensive adjustment, patients with persistent angina exhibited more than a 2-fold increased risk of cardiac death, myocardial infarction, and admission for heart failure.
Interestingly, angina class at inclusion (before treatment intervention) did not sufficiently stratify patient risk in the following months. However, as early as 6 months later, the presence of persistent angina emerged as an excellent predictor. Our findings are in agreement with the recent “Prospective Observational Longitudinal Registry of Patients with Stable Coronary Artery Disease” (CLARIFY registry), which showed that persistent angina at 1 and 5 years in 7212 medically managed patients was independently associated with higher rates of cardiovascular death or myocardial infarction.8,19
Overall, this reflects the importance of monitoring CCS patient symptoms not only at presentation but also after therapeutic interventions (both medical and/or invasive). The resolution of angina in the following months predicts a low probability of hard events, while the persistence of angina during this phase can help identify a high-risk subgroup requiring closer surveillance and individualized decision-making.
Revascularization in CCS patients has been a topic of ongoing debate in recent years.1,3,4,20,21 Regarding symptoms, and aside from more ambitious goals such as reducing the risk of hard events, it is important to remember that the main reason most CCS patients seek medical attention is angina pectoris.2 Our results indicate that in symptomatic patients with evident and extensive ischemic burden due to flow-limiting coronary lesions, a strategy based on CMR for detection, localization, and guidance of revascularization is effective in relieving angina symptoms. Indeed, the occurrence of persistent angina in our series was as low as 19%.
The findings of the present study, derived from stress CMR, concur with those of the recently published ORBITA-2 trial, which clearly demonstrated that in patients with objective evidence of ischemia from pressure wire, PCI results in a lower angina symptom score than a placebo procedure.5
This symptomatic improvement may be explained by a decrease in ischemic burden after revascularization. Indeed, the nuclear substudy of the COURAGE trial3 showed that PCI led to significant ischemia reduction and symptom relief, mainly in patients with large areas of ischemic myocardium (≥10%), where the amount of ischemic myocardium was significantly reduced. This rationale may also apply to our study, suggesting that the resolution or reduction of ischemic burden could explain the lower event rate observed in patients without persistent angina. Unfortunately, follow-up stress CMR studies were not regularly scheduled, and thus the mechanisms underlying the benefit of treatment intervention on the symptomatic improvement of our patients are speculative.
It is important to note that our study was purely observational and focused on the prognostic role of persistent angina after PCI revascularization. The efficacy of the applied strategy (namely, stress CMR for diagnosis and PCI guidance) compared with other approaches should be tested, if appropriate, in specifically designed trials.
Study limitationsThe registry database was designed with a limited number of variables to obtain a large cohort over an extended period, thereby avoiding missing values and maximizing the robustness of data collection. However, the availability of additional data, including more comprehensive scales for quantifying angina symptoms, would have allowed further collateral analyses, which constitutes a limitation of the study.
The long inclusion period and subsequent changes in recommendations and drug prescription may have influenced the patients’ prognosis. Thus, confirmation of our results in a prospective cohort of patients included over a shorter period and managed with up-to-date recommendations would be desirable.
Our study protocol included stress CMR, angiography, and percutaneous revascularization in all patients. We cannot exclude the possibility that these criteria introduced a certain entry bias, leading to an underrepresentation of patients with reduced systolic function, unfavorable coronary anatomy, frailty, or unstable conditions.
We did not systematically determine postrevascularization necrosis markers and, therefore, could not assess the prognostic impact of periprocedural infarctions.
Defining the most appropriate management to alleviate persistent angina after treatment intervention in CCS patients is beyond the scope of this study and would require further research, including a prospective randomized trial.
An exhaustive analysis of coronary physiology and the performance of follow-up angiograms (which were not routinely performed) might have helped clarify the ultimate cause of persistent angina in the 20 patients (32%) with no clear cause of this symptom.
CONCLUSIONSIn CCS patients with robust diagnostic evidence based on symptoms, stress CMR, and angiography, persistent angina in the months following percutaneous revascularization is a strong predictor of subsequent cardiac death, myocardial infarction, and heart failure admissions. Further research is needed to determine the optimal management of persistent angina in these patients.
- •
Management of angina symptoms in patients with CCS has been a topic of ongoing debate in recent years.
- •
This issue requires assessment in a homogeneous cohort using strict clinical, cardiac imaging, and angiographic inclusion criteria.
- •
In a registry of CCS patients with limiting angina symptoms, significant perfusion deficits on stress CMR, and severe coronary lesions in angiography, sustained improvement of angina symptoms was achieved in most patients after medical treatment optimization plus CMR-guided PCI.
- •
Persistent angina at 6 months after PCI was associated with higher rates of subsequent cardiac death, myocardial infarction, and admission for heart failure.
This work was supported by Instituto de Salud Carlos III and Fondos Europeos de Desarrollo Regional FEDER (grant numbers PI23/01150, CIBERCV16/11/00486, CIBERCV16/11/00403, CIBERCV16/11/00261, CIBERCV16/11/00420, CB16/11/00360), and by Conselleria de Educación – Generalitat Valenciana (PROMETEO/2021/008).
ETHICAL CONSIDERATIONSOur registry was conducted in accordance with the Helsinki Declaration. Events were collected retrospectively. In September 2018, the local ethics committee approved the study and a retrospective review of events occurring in the patients included in the registry, exempting the need for informed consent. Authorized personnel performed this review using the electronic regional health system registry from October to November 2018. Sex/gender biases were considered in the preparation of this paper in line with the SAGER guidelines
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence was used in the preparation of this paper.
AUTHORS’ CONTRIBUTIONSN. Pérez-Sole and E. de Dios contributed equally to this work. N. Pérez-Sole and E. de Dios collected the data, performed the analysis, wrote the paper, and revised the final manuscript. J.V. Monmeneu, M.P. López-Lereu, J. Gavara, C. Ríos-Navarro, V. Marcos-Garcés, H. Merenciano, C. Bonanad, F. Platero, and A. Ventura collected the data and revised the final manuscript.
J. Cánoves collected the data, secured funding, wrote the paper, and revised the final manuscript.
D. Moratal, A. Bayés-Genís, J. Sanz, M. Jiménez-Navarro, L. Martínez-Dolz, and J. Sanchis collected the data and revised the final manuscript. V. Bodi conceived and designed the analysis, secured funding, wrote the paper, and revised the final manuscript.
CONFLICTS OF INTERESTJ. Sanchis is editor-in-chief of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed. No other conflicts of interest exist in the study.