We sought to assess the prognostic value of exercise-induced left ventricular systolic dysfunction in hypertensive patients with normal resting echocardiography and absence of coronary artery disease.
MethodsFrom our database of patients referred for treadmill exercise echocardiography, we identified 93 hypertensive patients with preserved resting left ventricular ejection fraction (≥ 50%), no evidence of structural heart disease, and absence of coronary artery disease on angiography. Overall, 39 patients developed exercise-induced left ventricular systolic dysfunction (defined as a decrease in left ventricular ejection fraction below 50% at peak exercise) and 54 exhibited a normal left ventricular ejection fraction response to exercise. The mean follow-up was 6.1 (3.7) years. End points were all-cause mortality, cardiac death, heart failure, and the composite event of cardiac death or heart failure.
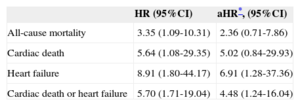
ResultsPatients who developed exercise-induced left ventricular systolic dysfunction were at higher risk of death from any cause (hazard ratio=3.4; 95% confidence interval, 1.1-10.3), cardiac death (hazard ratio=5.6; 95%CI, 1.1-29.4), heart failure (hazard ratio=8.9; 95% confidence interval, 1.8-44.2), and the composite end point (hazard ratio=5.7; 95% confidence interval, 1.7-19.0). In the multivariate analysis, exercise-induced left ventricular systolic dysfunction remained an independent predictor of both heart failure (hazard ratio=6.9; 95% CI, 1.3-37.4) and the composite event of cardiac death or heart failure (hazard ratio=4.5; 95% confidence interval, 1.2-16.0).
ConclusionsIn hypertensive patients with preserved resting left ventricular ejection fraction and absence of coronary artery disease, exercise-induced left ventricular systolic dysfunction is a strong predictor of cardiac events and may represent early hypertensive heart disease.
Keywords
Hypertension is one of the main risk factors for the development of left ventricular hypertrophy (LVH), coronary artery disease (CAD), and heart failure (HF).1–3 Patients with high blood pressure have a 2- to 3-fold risk for HF compared with normotensive subjects, as shown by data from the Framingham Heart Study.4 The development of hypertensive heart disease is characterized by an initial period of latent remodeling, consisting of cardiomyocyte hypertrophy, interstitial fibrosis, altered cell metabolism, and microvascular disease, among other alterations. In this regard, the time at which LVH becomes evident in echocardiography probably represents an advanced stage of myocardial damage.5,6 Therefore, there is a need for early detection of changes in ventricular structure and function to prevent or delay irreversible tissue injury and consequent HF onset.
Hypertensive patients with normal standard resting echocardiography may develop abnormalities of diastolic and systolic left ventricular function during exercise. Early studies demonstrated that a decrease in left ventricular ejection fraction (LVEF) may occur during exercise in patients with mild-to-moderate hypertension and absence of LVH or CAD.7 More recent studies have also demonstrated impaired long-axis function, and left ventricular twist and suction.8 All these abnormal ventricular functional changes, which are apparent only on exercise, might represent the earliest changes in hypertensive heart disease. However, the clinical implications of these findings have not been characterized so far.
The aim of this study was to assess the prognostic value of exercise-induced left ventricular systolic dysfunction (EILVD) in hypertensive patients with normal results on resting echocardiography and absence of angiographic CAD. We hypothesized that a depressed LVEF response to exercise may be an early indicator of cardiac damage identifying hypertensive patients at risk of developing HF and cardiac events.
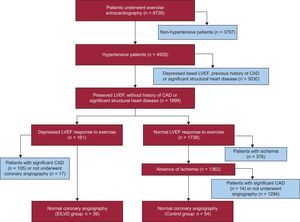
METHODSPatient SelectionWe screened 8726 consecutive patients who underwent exercise echocardiography for clinical reasons at our institution from November 1, 1997 to August 31, 2009. Hypertensive patients with normal resting LVEF were identified (n = 4217). Hypertension was defined as resting blood pressure > 140/90mmHg or a previously established diagnosis. Normal resting ventricular function was considered if LVEF was ≥ 50%. We excluded patients with a history of ischemic heart disease or significant valve disease (more than mild valvular stenosis or regurgitation) and those with more than mild LVH (septal thickness or posterior wall thickness >12mm for women and >13mm for men).9 Among the remaining patients (n=1899), we identified those who developed EILVD and who underwent a subsequent coronary angiography showing the absence of significant CAD (EILVD group; n=39). Exercise-induced left ventricular systolic dysfunction was defined as a decrease in LVEF below 50% at peak exercise. The control group comprised 54 consecutive hypertensive patients evaluated within the same time period and with true negative exercise echocardiographic studies (ie, patients with a normal LEFV response and absence of ischemia during exercise and who underwent angiography within 3 months showing unobstructed coronary arteries). Figure 1 represents a flowchart of patients included in the study.
The demographic and clinical data and stress testing results were entered in our prospective database at the time of the procedures. All patients signed an informed consent form before testing.
Exercise Treadmill TestingHeart rate, blood pressure, and a 12-lead resting electrocardiogram were obtained at each stage of the exercise protocol. Patients were encouraged to perform a treadmill exercise test adjusted to each patient's characteristics (Bruce protocol 90.3%; modified Bruce 6.5%, modified Bruce for sportspersons 2.2%, Naughton 1.1%) until they reached an end point. Exercise end points included physical exhaustion, significant arrhythmia, severe hypertension (systolic blood pressure > 240mmHg or diastolic blood pressure > 110mmHg), severe angina, and severe hypotensive response (decrease > 20mmHg in systolic blood pressure from baseline). Ischemic electrocardiogram abnormalities during the test were defined as the development of ST-segment deviation of ≥ 1mm 80ms after the J point. A hypertensive response to exercise was defined as a maximum systolic/diastolic blood pressure ≥ 210/105mm Hg in men and ≥ 190/105mm Hg in women.10
Exercise Echocardiography and Imaging AnalysisTwo-dimensional echocardiography was performed in 3 apical views (4-chamber, 2-chamber, and 3-chamber) and 2 parasternal views (long- and short-axis) at rest, peak exercise, and in the immediate postexercise period. Peak and postexercise images were obtained using a continuous imaging capture system, the former with the patient still exercising, the second with the patient lying on the table. Peak imaging was performed when signs of exhaustion were present or an end point was reached, as previously described.11 Echocardiographic analysis was performed on a digital quad screen display system to allow comparison of the same planes at rest and on exercise. LVEF was visually assessed at rest, peak exercise, and postexercise.12,13 The change in LVEF from rest to peak exercise (¿ LVEF) was calculated. Regional wall motion was also evaluated with a 16-segment model of the left ventricle.14 Each segment was graded on a 4-point scale, with normal wall motion scoring = 1; hypokinesia = 2; akinesia = 3, and dyskinesia = 4. The wall motion score index was calculated at rest, peak exercise, and postexercise as the sum of the scores divided by the number of segments evaluated. The worst LVEF and wall motion score index obtained at peak or postexercise were considered. The change in wall motion score index from rest to peak exercise was also calculated. Ischemia was defined as the development of new or worsening wall motion abnormalities with exercise, except isolated hypokinesia of the inferobasal segment, which was not considered abnormal, except when an adjacent segment was also abnormal.15 Extensive ischemia was defined as wall motion abnormalities involving at least 3 myocardial segments. Global ischemia was defined as extensive ischemia involving at least 2 different coronary territories.
Patients included in this study had good imaging quality, allowing evaluation of ≥ 15 segments both at rest and during and/or immediately after exercise in all of them. The feasibility and accuracy of peak imaging during treadmill exercise have been reported previously.16
Coronary AngiographyAll patients included in the study underwent coronary angiography for clinical reasons. Procedures were performed and interpreted by highly experienced interventional cardiologists. Angiographically significant CAD was defined as the presence of at least 1 stenosis of ≥ 50% diameter in any of the epicardial coronary arteries or major branches.
Follow-up and End PointsFollow-up and identification of events were carried out by reviewing hospital databases, medical records, and death certificates, as well as by telephone interviews when necessary. Patients were assessed for all-cause mortality, cardiac death, the development of HF, and the composite end point of cardiac death and HF. Cardiac death was defined as death due to HF, acute myocardial infarction, arrhythmia, or cardiac arrest; unexpected or unexplained sudden death was also considered cardiac death. Heart failure was considered in cases of hospitalization because of new-onset HF. We also evaluated follow-up echocardiograms performed during follow-up in patients who had HF to identify those who developed reduced left ventricular ejection fraction (resting LVEF <50%).
Acute myocardial infarction was defined as the appearance of new symptoms of myocardial ischemia or ischemic electrocardiogram changes accompanied by an increase in markers of myocardial injury. Revascularization procedures during follow-up were also collected.
Statistical AnalysisResults are presented as mean (standard deviation [SD]) for continuous normally-distributed variables, as median [interquartile range] for continuous non-normally-distributed data, and as percentages for categorical data. Analysis of normality was performed with the Kolmogorov–Smirnov and Shapiro-Wilk test. Categorical data were compared using the chi-square-test or Fisher exact test, as required. Comparisons of continuous variables were analyzed using Student's unpaired t-test and the Mann–Whitney U-test, as appropriate.
Cumulative event curves were estimated by the Kaplan-Meier method and compared by the log-rank test. Patients were censored at the time of HF onset or death for the combined analysis. Univariate and multivariate associations were assessed with Cox proportional hazards models. Variables were selected in a stepwise forward selection manner, with entry and retention set at a significance level of 0.05. The variables used in the multivariate model were age, diabetes mellitus, resting LVEF, and resting systolic blood pressure. Hazard ratios with 95% confidence intervals (95% CI) were estimated. Statistical analyses were performed with the use of SPSS software (version 17.0, SPSS Inc.; Chicago, Illinois, United Stated).
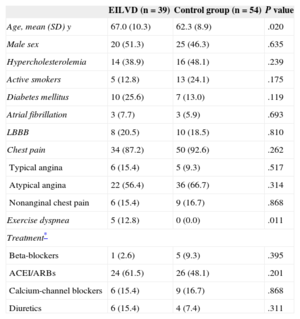
RESULTSBaseline and Exercise Echocardiographic CharacteristicsThe mean age was 64.3 (9.7) years, and 45 patients (48.4%) were men. The clinical and demographic characteristics of the patients according to LVEF response to exercise are summarized in Table 1. Exercise echocardiography data are shown in Table 2. Clinical baseline variables were similar between groups, with the exception that patients with EILVD were slightly older and were more likely to have exercise dyspnea. Overall, the main reason for referring patients in both groups to exercise echocardiography was chest pain.
Baseline Clinical Characteristics
| EILVD (n=39) | Control group (n=54) | P value | |
|---|---|---|---|
| Age, mean (SD) y | 67.0 (10.3) | 62.3 (8.9) | .020 |
| Male sex | 20 (51.3) | 25 (46.3) | .635 |
| Hypercholesterolemia | 14 (38.9) | 16 (48.1) | .239 |
| Active smokers | 5 (12.8) | 13 (24.1) | .175 |
| Diabetes mellitus | 10 (25.6) | 7 (13.0) | .119 |
| Atrial fibrillation | 3 (7.7) | 3 (5.9) | .693 |
| LBBB | 8 (20.5) | 10 (18.5) | .810 |
| Chest pain | 34 (87.2) | 50 (92.6) | .262 |
| Typical angina | 6 (15.4) | 5 (9.3) | .517 |
| Atypical angina | 22 (56.4) | 36 (66.7) | .314 |
| Nonanginal chest pain | 6 (15.4) | 9 (16.7) | .868 |
| Exercise dyspnea | 5 (12.8) | 0 (0.0) | .011 |
| Treatment* | |||
| Beta-blockers | 1 (2.6) | 5 (9.3) | .395 |
| ACEI/ARBs | 24 (61.5) | 26 (48.1) | .201 |
| Calcium-channel blockers | 6 (15.4) | 9 (16.7) | .868 |
| Diuretics | 6 (15.4) | 4 (7.4) | .311 |
ACEI, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor antagonists; EILVD, exercise-induced left ventricular systolic dysfunction; LBBB, left bundle branch block.
Data are expressed as n (%) or mean (standard deviation).
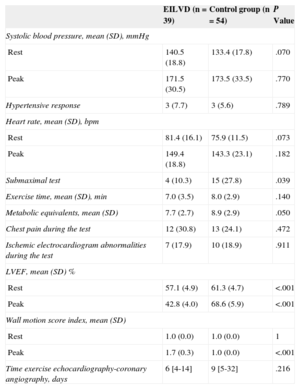
Exercise Echocardiographic Data
| EILVD (n = 39) | Control group (n = 54) | P Value | |
|---|---|---|---|
| Systolic blood pressure, mean (SD), mmHg | |||
| Rest | 140.5 (18.8) | 133.4 (17.8) | .070 |
| Peak | 171.5 (30.5) | 173.5 (33.5) | .770 |
| Hypertensive response | 3 (7.7) | 3 (5.6) | .789 |
| Heart rate, mean (SD), bpm | |||
| Rest | 81.4 (16.1) | 75.9 (11.5) | .073 |
| Peak | 149.4 (18.8) | 143.3 (23.1) | .182 |
| Submaximal test | 4 (10.3) | 15 (27.8) | .039 |
| Exercise time, mean (SD), min | 7.0 (3.5) | 8.0 (2.9) | .140 |
| Metabolic equivalents, mean (SD) | 7.7 (2.7) | 8.9 (2.9) | .050 |
| Chest pain during the test | 12 (30.8) | 13 (24.1) | .472 |
| Ischemic electrocardiogram abnormalities during the test | 7 (17.9) | 10 (18.9) | .911 |
| LVEF, mean (SD) % | |||
| Rest | 57.1 (4.9) | 61.3 (4.7) | <.001 |
| Peak | 42.8 (4.0) | 68.6 (5.9) | <.001 |
| Wall motion score index, mean (SD) | |||
| Rest | 1.0 (0.0) | 1.0 (0.0) | 1 |
| Peak | 1.7 (0.3) | 1.0 (0.0) | <.001 |
| Time exercise echocardiography-coronary angiography, days | 6 [4-14] | 9 [5-32] | .216 |
EILVD, exercise-induced left ventricular systolic dysfunction; LVEF, Left ventricular ejection fraction.
Data are expressed as no. (%), mean (standard deviation) or median [interquartile range].
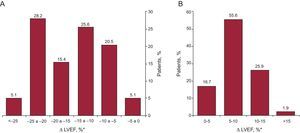
With regard to exercise echocardiography data, patients with EILVD had lower LVEF at rest and at exercise. Most patients in this group experienced a marked drop in LVEF with exercise: 29 (74.4%) showed a reduction of ≥ 10 units and 13 (33.3%) showed a reduction of ≥ 20 units (Figure 2A). In contrast, most of the patients without EILVD experienced an increase in LVEF of at least 5 units (45 patients [83.3%]) (Figure 2B). No patients in the control group had wall motion abnormalities during exercise, whereas either extensive or global ischemia was seen in most of the patients with EILVD (36 patients [92.3%]). As expected, the wall motion score index was significantly higher in the exercise images of patients with EILVD, whereas metabolic equivalents were lower. There were no differences between groups in symptoms or electrocardiogram changes during exercise. The frequency of hypertensive response was also similar between patients with and without EILVD.
Change in left ventricular ejection fraction from rest to peak exercise. A: group of patients that exhibited exercise-induced left ventricular systolic dysfunction. B: patients with normal left ventricular ejection fraction response to exercise (control group). LVEF, left ventricular ejection fraction. *Left ventricular ejection fraction from rest to peak exercise.
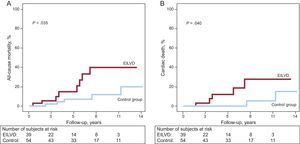
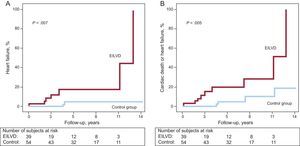
Among the 93 patients included, there were 13 (14.0%) deaths and 9 (9.7%) patients developed new-onset HF during the follow-up (at a mean of 6.1 [3.7] years). The cause of death was identified as cardiac in 7 patients (53.8%). When compared by groups, the development of EILVD was associated with significantly higher rates of death from any cause (Figure 3A), cardiac death (Figure 3B), HF (Figure 4A), and the composite end point of cardiac death or HF (Figure 4B). There were 8 (20.5%) deaths in the EILVD group (5 were cardiac deaths) and 5 (9.3%) deaths in the control group (2 cardiac deaths). Focusing on HF, 7 (17.9%) patients in the EILVD group were hospitalized for new-onset HF, including 5 (12.8%) patients that developed resting LVEF systolic dysfunction during follow-up, whereas the incidence of HF in the control group was 3.7% (2 patients), including only 1 (1.9%) patient with reduced ejection fraction at rest.
In the multivariate analysis, with the aforementioned variables, EILVD remained an independent predictor of both HF (adjusted hazard ratio [HR] = 6.9; 95%CI, 1.3-37.4) and the composite event of cardiac death or HF (HR = 4.5; 95%CI, 1.2-16.0). Furthermore, the multivariate analysis suggested an association between EILVD and cardiac death; however, this result was not statistically significant (HR = 5.0; 95%CI, 0.8-29.9). Hazard ratios for the distinct events, both in the univariate and multivariate analyses, are shown in Table 3.
Exercise-induced Left Ventricular Systolic Dysfunction and Clinical Events. Hazard Ratios in the Univariate and Multivariate Analyses
| HR (95%CI) | aHR*, (95%CI) | |
|---|---|---|
| All-cause mortality | 3.35 (1.09-10.31) | 2.36 (0.71-7.86) |
| Cardiac death | 5.64 (1.08-29.35) | 5.02 (0.84-29.93) |
| Heart failure | 8.91 (1.80-44.17) | 6.91 (1.28-37.36) |
| Cardiac death or heart failure | 5.70 (1.71-19.04) | 4.48 (1.24-16.04) |
95%CI, 95% confidence interval; aHR: adjusted hazard ratio; HR: hazard ratio.
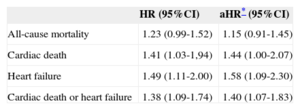
There was also a significant relationship between the magnitude of change in LVEF (¿ LVEF) during exercise and clinical outcomes (Table 4). In both the univariate and multivariate analyses, every 5-unit decrease in LVEF was associated with a 41% (HR = 1.41; 95%CI, 1.03-1.94) and 44% (HR = 1.44; 95%CI, 1.00-2.07) increased risk of cardiac death, respectively. For HF, a 49% (HR = 1.49; 95%CI, 1.11-2.00) and 58% (HR = 1.58; 95%CI, 1.09-2.30) increased risk were observed in the univariate and multivariate models, respectively, for each 5-unit decrease in LEVF on exercise.
Outcome Hazard Ratios Associated With Every 5-Unit Decrease in Left Ventricular Ejection Fraction During Exercise
| HR (95%CI) | aHR* (95%CI) | |
|---|---|---|
| All-cause mortality | 1.23 (0.99-1.52) | 1.15 (0.91-1.45) |
| Cardiac death | 1.41 (1.03-1,94) | 1.44 (1.00-2.07) |
| Heart failure | 1.49 (1.11-2.00) | 1.58 (1.09-2.30) |
| Cardiac death or heart failure | 1.38 (1.09-1.74) | 1.40 (1.07-1.83) |
95%CI, 95% confidence interval; aHR: adjusted hazard ratio; HR: hazard ratio.
Lastly, apart from 1 patient in the EILVD group who had a myocardial infarction, no other patients in the study had an acute coronary event or showed evidence of CAD during follow-up.
DISCUSSIONTo the best of our knowledge, the present study is the first to assess the prognostic implications of EILVD in hypertensive patients. Our study demonstrates that patients with high blood pressure who exhibited a depressed LVEF response to exercise, even without significant LVH or angiographic CAD, were at increased risk of new-onset HF and cardiac death. These findings highlight the role of exercise echocardiography in the detection of early hypertensive heart disease and provide significant prognostic information for the prediction of cardiac events in these patients.
Exercise-induced Left Ventricular Systolic Dysfunction in Hypertensive Patients: Previous Work and Prognostic ImplicationsIt has been previously shown that abnormalities of left ventricular systolic function that are apparent only during exercise may occur in hypertensive patients with normal resting echocardiography and without evidence of CAD. Tan et al8 found that in patients with well-treated hypertension without LVH there were abnormalities of longitudinal function, twist, and strain that all worsened during exercise. A similar response to exercise has been reported in patients with HF with normal ejection fraction, most of them with history of hypertension.17 Moreover, it has been long observed that hypertension may be associated with abnormalities in LVEF response to exercise, despite no evidence of CAD. Several studies have shown that hypertensive patients may show alterations ranging from a lesser increase in LVEF as compared with normotensive patients to a mild-to-moderate decrease in LVEF during exercise.8,18 However, the clinical implications of these findings have not been previously determined. The present work complements and expands previous studies by demonstrating that hypertensive patients without significant LVH may develop a marked decrease in LVEF during exercise even in the absence of CAD. In addition, for the first time we report a significant association between this response and hard cardiac events. In fact, the annualized mortality and HF rates were > 3% in hypertensive patients who developed EILVD; moreover, in this group of patients, the HF rate was almost 5 times higher than in the group of patients with a normal LVEF exercise response, and the cardiac death rate was 3.5 higher. Furthermore, our results highlight the importance of the magnitude of change in ¿ LVEF during exercise: the greater the decrease in LVEF, the worse the prognosis.
Mechanisms of Exercise-induced Left Ventricular DysfunctionThe main mechanism of EILVD in hypertensive patients without coronary disease remains unclear. An exaggerated blood pressure response to exercise has been postulated as a leading cause of left ventricular function abnormalities in the absence of CAD.19,20 However, a recent study did not find that a marked increase in systolic blood pressure was associated with an abnormal exercise echocardiographic result.21 Consistent with this latter study, in our cohort of patients, the frequency of a hypertensive response to exercise was low and was not predictive of EILVD development.
On the other hand, ultrastructural myocardial alterations occurring in response to chronic pressure overload may play a fundamental role in EILVD in hypertensive patients. Coronary microvascular endothelial dysfunction is an early event in hypertensive heart disease and has been suggested to be involved in the development of HF.22,23 Perivascular fibrosis may also contribute to myocardial perfusion abnormalities and subsequent myocardial damage.24,25 Furthermore, previous studies have shown that, even in the absence of LVH, hypertensive patients have impaired myocardial metabolism, which worsens during pharmacological stress testing.26 Therefore, we believe that in the early stages of hypertensive heart disease, these intrinsic myocardial alterations, including microvascular coronary dysfunction and impaired myocardial metabolism, could explain the development of abnormalities of left ventricular systolic function that are evident only during exercise, when myocardial oxygen demand is greatest.
Certain issues of the present study deserve mention and may support the above hypothesis. Patients who developed EILVD had a lower resting LVEF (at the lower limit of normal) and lower exercise capacity (based on metabolic equivalents) than the control group. These findings might be an expression of subtle incipient myocardial disease, which would have gone unnoticed if exercise echocardiography had not been performed. Moreover, hypertensive patients with a depressed LVEF response to exercise were at particularly increased risk of new-onset HF, mostly related to the development of left ventricular systolic dysfunction. In contrast, CAD-related events were extremely infrequent during follow-up. All these findings suggest that EILVD may represent an early marker of structural myocardial damage in hypertensive patients, and support the role of exercise echocardiography in identifying patients at risk of developing patent hypertensive heart disease and symptomatic HF.
Finally, in line with previous work, the abnormalities of left ventricular systolic function observed during exercise occurred even in the absence of significant LVH.7 Several studies have revealed that adaptive hypertrophy is but one of many structural changes in hypertensive heart disease, and it remains unclear whether LVH is a pathogenic step in the development of systolic HF or should be regarded as an epiphenomenon to cardiac overload.5,27 In fact, data from human studies showed that concentric hypertrophy does not frequently progress to systolic dysfunction in the absence of coronary disease.28,29
Study LimitationsThis is an observational study, and consequently there could be uncontrollable confounding factors that could account for at least part of the observed differences between groups. The study population consisted of a selected subgroup of hypertensive patients referred to exercise echocardiography and who underwent subsequent coronary angiography for clinical reasons; consequently, the results may not be applicable to all hypertensive patients. Given that the results of the test were available to treating physicians, patients with a depressed LVEF response to exercise may have been more likely to receive more intensive medical therapy and to undergo closer follow-up; thus, the actual prognostic impact of EILVD in hypertensive patients may have been significantly underestimated. In addition, the medication change after testing and its effect on outcomes could not be assessed. Equally, the ascertainment of cause of death may be susceptible to bias and misclassification.30 Furthermore, we cannot estimate the true incidence of the development of a depressed LVEF because a follow-up echocardiogram was performed only if clinically indicated.
For the purpose of the present study, LVEF was visually assessed. Visually estimated LVEF has been reported to be closely correlated with quantitative determination of LVEF13 and is a predictor of mortality and cardiac events in other populations.11 However, our results could have been different if we had used quantitative measurements. Finally, we performed imaging acquisition at peak exercise because it has higher sensitivity for detecting CAD.16 This technique is not widely employed, and our results might have differed if we had acquired images only after exercise.
CONCLUSIONSThe present results indicate that hypertensive patients with preserved LVEF and without significant LVH who develop EILVD despite the absence of CAD are at increased risk of progression to clinical HF and cardiac death. Although further studies will be needed to demonstrate our findings conclusively and to elucidate the optimal management of these patients, we consider that patients with a depressed LVEF response to exercise should undergo close follow-up and strict hypertension control, emphasizing the importance of using agents that may prevent or reverse these early abnormalities of ventricular function in order to avoid cardiac events.
CONFLICTS OF INTERESTNone declared.