To identify the current mortality and management of patients admitted for suspected acute coronary syndrome in Spain. The last available registry (2004-2005) reported an in-hospital mortality of 5.7%.
MethodsThe study included patients consecutively admitted between January and June 2012 at 44 hospitals selected at random. Information was collected on clinical course at admission and on events at 6 months.
ResultsA total of 2557 patients admitted with suspected acute coronary syndrome were included: 788 (30.8%) with ST-segment elevation, 1602 (62.7%) without ST-segment elevation, and 167 (6.5%) with unclassified acute coronary syndrome. In-hospital mortality was 4.1% (6.6%, 2.4%, and 7.8% respectively), significantly lower than that observed for 2004-2005. Reperfusion treatment (most commonly, primary percutaneous coronary intervention) was administered to 85.7% of patients with ST-segment elevation attended within 12h. The median time from first medical contact to thrombolysis was 40min and to balloon inflation, 120min. Among patients without ST-segment elevation, coronary angiography was performed in 80.6%, percutaneous intervention in 52.0%, and surgery was indicated in 6.4%. Secondary prevention treatments at discharge was prescribed more often than in earlier registries. In patients alive at discharge (follow-up available for 97.1%), 6-month mortality was 3.8%.
ConclusionsMortality among patients with acute coronary syndrome in Spain was lower than that reported in the most recent published studies, in parallel with a more frequent use of the main treatments recommended.
Keywords
Acute coronary syndrome (ACS) is the main complication of ischemic heart disease and has considerable health impact.1,2 In Spain, several ACS registries3–7 have investigated the prognosis and management of the condition and its clinical course over time.8
The MASCARA study7 included patients from 2004 to 2005 and is the last of these large registries. Since then, ACS management has seen several changes, such as the widespread use of reperfusion therapies for ST-segment elevation ACS (STEACS) or primary percutaneous coronary intervention (PCI) as a reperfusion technique, the introduction of new drugs, or the popularization of radial access for coronary angiography. These changes have been included in the clinical practice guidelines9,10 and explain the rationale for collecting up-to-date information on the prognosis and management of ACS.
In Spain, the DIOCLES (Descripción de la Cardiopatía Isquémica en el Territorio Español) registry, sponsored by the Sección de Cardiopatía Isquémica y Cuidados Agudos Cardiovasculares of the Sociedad Española de Cardiología and the Sociedad Española de Medicina Intensiva, Crítica y de Unidades Coronarias, investigated in-hospital and 6-month mortality among patients admitted for suspected ACS and described their care management.
METHODSStudy DesignThis multicenter, observational, cross-sectional study prospectively collected admission data and performed 6-month follow-up among patients ≥ 18 subsequently admitted for suspected ACS that was first managed at the participating site (except prehospital treatment or admission a few hours after primary PCI at another site) and who gave written consent. Consent was not required to analyze cases of in-hospital death. Patients were excluded if ACS was secondary to other processes, such as tachyarrhythmia, severe anemia, or surgery; if they had been transferred from another site where they had been admitted for ACS; and if they had recently participated in another clinical trial.
Data were collected on demographic variables, risk factors, disease history, and clinical presentation; prehospital, hospital, and discharge management; complications; and in-hospital mortality (eg, death during admission at the participating hospital). At 6 months, information was collected by a centralized phone interview or by the local investigator regarding the occurrence of death (and its cause), acute myocardial infarction, stroke, coronary revascularization or cardiovascular readmission, and the date of the event.
ACS was classified according to electrocardiographic presentation as STEACS (ST-segment elevation ≥ 1mm in ≥ 2 contiguous leads > 20 min), NSTEACS (all other patients with electrocardiogram interpretable as signs of ischemia), and unclassified ACS (noninterpretable electrocardiogram due to left bundle-branch block, pacemaker pacing, or Wolff-Parkinson-White syndrome).
Site Selection and ScheduleIn order to recruit 50 sites, 70 public or subsidized unspecialized hospitals with more than 50 beds registered in the Ministry of Health database were preselected at random. As in previous studies,4,5,7 randomization was stratified by health care level to include 35% sites with a cardiologic or general critical care unit and interventional cardiology laboratory (type A site), 45% with a critical care unit without interventional cardiology laboratory (type B site), and 20% without a critical care unit (type C site). Two sites were included by additional invitation.
Each site with 1 or more physicians involved in ACS management was contacted and agreed to identify patients, request informed consent, enter the admission information into an online form, and send the phone contact details to a company in charge of follow-up. When phone contact was not made, the investigators were asked to obtain follow-up information from the medical records. The study was approved by a lead ethics committee, by the ethics committees of the hospitals that required it, and by the relevant government agencies in the autonomous communities involved. A total of 70 hospitals were preselected, of which 50 were included; the remaining sites were not included mainly because no local investigator was found, administrative delays were encountered, or the upper limit of 50 sites had been reached. Among these 50, 6 were later excluded due to insufficient patient inclusion, such that the final study included 44 sites (18 of type A, 21 of type B, and 5 of type C). Patient enrollment was open between 10 January and 15 June 2012; however, not all hospitals participated during the entire period because some joined late for administrative reasons and others because they had met their participation quota. The mean time for which the hospitals remained active was 3.6 (1.2) months, with no significant differences between the 3 categories.
Quality Enhancement and ControlThe methodology was explained to the investigators via teleconference with slide presentations, and information letters were sent regularly. It was predetermined that sites that stopped recruitment or had clearly insufficient enrollment (< 5 patients) would be excluded. Monitoring visits were made to 8 sites in 6 autonomous communities; these sites were chosen at random to evaluate compliance with the ethical and legal aspects, as well as data consistency in the forms and medical records or ≥ 7 of 10 predefined variables. The results were favorable in all cases. Investigators were asked for a list of patients not included and the reasons. Validation filters and rules were established in numerous variables on the form. The company in charge of database design and operation and the principal investigators for the study (JAB and AB), responsible for the statistical analysis, monitored internal data consistency during the analysis phase, consulting investigators if any inconsistencies or possible errors were detected and making any necessary corrections.
Statistical AnalysisThe sample size required to estimate in-hospital patient mortality with 95% certainty and precision of±1% was calculated based on a predicted mortality of 5.7%, which was the mortality observed in the MASCARA registry.7 It was assumed that 20% of patients would be nonassessable and, therefore, it was considered necessary to include 2581 patients.
The continuous variables are expressed as median [interquartile range] and the categorical variables as percentages. The Kruskal-Wallis test was used to compare continuous variables and the chi-square test to compare categorical variables. Kaplan-Meier survival curves were prepared and compared by the log rank test statistic. All analyses were performed using SPSS 13.0.
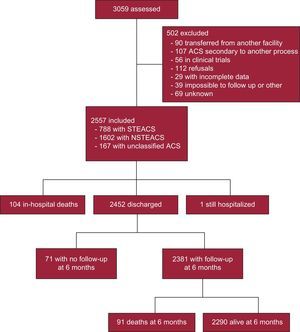
ResultsBaseline Characteristics and Clinical PresentationThe study included 44 sites in 13 autonomous communities (all except for Balearic Islands, Canary Islands, Castile-LaMancha, and La Rioja, which are not represented because no hospitals in these communities were selected at random or due to administrative delays). A total of 3059 patients were assessed, and 502 of these were excluded for the reasons described in Figure 1. Therefore, the study included 2557 patients: 788 (30.8%) with an admission diagnosis of STEACS, 1602 (62.7%) with NSTEACS, and 167 (6.5%) with unclassified ACS.
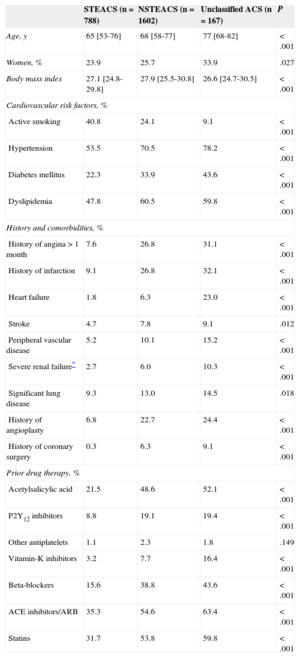
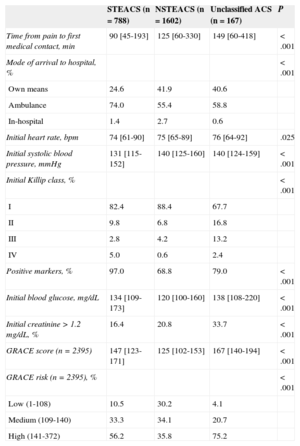
Table 1 summarizes the baseline data according to type of ACS. When compared to patients with STEACS, patients with NSTEACS or unclassified ACS were older, were more likely to be women, had a higher prevalence of cardiovascular risk factors except for smoking, comorbidities, and history of ischemic heart disease, and were more frequent users of cardiovascular drugs.
Demographic and Clinical Characteristics According to Diagnosis on Admission
| STEACS (n=788) | NSTEACS (n=1602) | Unclassified ACS (n=167) | P | |
|---|---|---|---|---|
| Age, y | 65 [53-76] | 68 [58-77] | 77 [68-82] | <.001 |
| Women, % | 23.9 | 25.7 | 33.9 | .027 |
| Body mass index | 27.1 [24.8-29.8] | 27.9 [25.5-30.8] | 26.6 [24.7-30.5] | <.001 |
| Cardiovascular risk factors, % | ||||
| Active smoking | 40.8 | 24.1 | 9.1 | <.001 |
| Hypertension | 53.5 | 70.5 | 78.2 | <.001 |
| Diabetes mellitus | 22.3 | 33.9 | 43.6 | <.001 |
| Dyslipidemia | 47.8 | 60.5 | 59.8 | <.001 |
| History and comorbidities, % | ||||
| History of angina>1 month | 7.6 | 26.8 | 31.1 | <.001 |
| History of infarction | 9.1 | 26.8 | 32.1 | <.001 |
| Heart failure | 1.8 | 6.3 | 23.0 | <.001 |
| Stroke | 4.7 | 7.8 | 9.1 | .012 |
| Peripheral vascular disease | 5.2 | 10.1 | 15.2 | <.001 |
| Severe renal failure* | 2.7 | 6.0 | 10.3 | <.001 |
| Significant lung disease | 9.3 | 13.0 | 14.5 | .018 |
| History of angioplasty | 6.8 | 22.7 | 24.4 | <.001 |
| History of coronary surgery | 0.3 | 6.3 | 9.1 | <.001 |
| Prior drug therapy, % | ||||
| Acetylsalicylic acid | 21.5 | 48.6 | 52.1 | <.001 |
| P2Y12 inhibitors | 8.8 | 19.1 | 19.4 | <.001 |
| Other antiplatelets | 1.1 | 2.3 | 1.8 | .149 |
| Vitamin-K inhibitors | 3.2 | 7.7 | 16.4 | <.001 |
| Beta-blockers | 15.6 | 38.8 | 43.6 | <.001 |
| ACE inhibitors/ARB | 35.3 | 54.6 | 63.4 | <.001 |
| Statins | 31.7 | 53.8 | 59.8 | <.001 |
ACEI, angiotensin-converting enzyme; ACS, acute coronary syndrome; ARB, angiotensin receptor blockers; NSTEACS, non–ST-segment elevation acute coronary syndrome; STEACS, ST-segment elevation acute coronary syndrome.
Unless otherwise indicated, data are expressed as median [interquartile range].
Table 2 summarizes the data on initial care and on arrival to the hospital. Patients with STEACS took less time to seek care, and three quarters were brought to the hospital by ambulance, whereas most patients in the other groups came on their own. Killip class IV status was more common in STEACS patients. The GRACE (Global Registry of Acute Coronary Events) score9 was higher in those patients than in patients with NSTEACS and was particularly high in those with unclassified ACS.
Early Care According to Admission Diagnosis
| STEACS (n=788) | NSTEACS (n=1602) | Unclassified ACS (n=167) | P | |
|---|---|---|---|---|
| Time from pain to first medical contact, min | 90 [45-193] | 125 [60-330] | 149 [60-418] | <.001 |
| Mode of arrival to hospital, % | <.001 | |||
| Own means | 24.6 | 41.9 | 40.6 | |
| Ambulance | 74.0 | 55.4 | 58.8 | |
| In-hospital | 1.4 | 2.7 | 0.6 | |
| Initial heart rate, bpm | 74 [61-90] | 75 [65-89] | 76 [64-92] | .025 |
| Initial systolic blood pressure, mmHg | 131 [115-152] | 140 [125-160] | 140 [124-159] | <.001 |
| Initial Killip class, % | <.001 | |||
| I | 82.4 | 88.4 | 67.7 | |
| II | 9.8 | 6.8 | 16.8 | |
| III | 2.8 | 4.2 | 13.2 | |
| IV | 5.0 | 0.6 | 2.4 | |
| Positive markers, % | 97.0 | 68.8 | 79.0 | <.001 |
| Initial blood glucose, mg/dL | 134 [109-173] | 120 [100-160] | 138 [108-220] | <.001 |
| Initial creatinine>1.2 mg/dL, % | 16.4 | 20.8 | 33.7 | <.001 |
| GRACE score (n=2395) | 147 [123-171] | 125 [102-153] | 167 [140-194] | <.001 |
| GRACE risk (n=2395), % | <.001 | |||
| Low (1-108) | 10.5 | 30.2 | 4.1 | |
| Medium (109-140) | 33.3 | 34.1 | 20.7 | |
| High (141-372) | 56.2 | 35.8 | 75.2 |
ACS, acute coronary syndrome; GRACE, Global Registry of Acute Coronary Events; NSTEACS, non—ST-segment elevation acute coronary syndrome; STEACS, ST-segment elevation acute coronary syndrome.
Unless otherwise indicated, data are expressed as median [interquartile range].
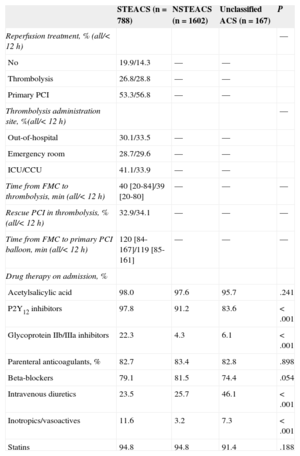
Table 3 illustrates patient treatment. A total of 80% of those with suspected STEACS (82% when excluding patients in whom ACS was not confirmed) received reperfusion, which was primary PCI in approximately 2 of 3 of patients. The median times between first medical contact and thrombolytic administration or balloon inflation were 40min and 120min, respectively. In 41% of cases, the thrombolytic therapy was administered in the critical care unit. Almost all patients received dual antiplatelet therapy and statins; anticoagulant use was somewhat lower, and glycoprotein IIb/IIIa inhibitor use was much lower and almost only seen in the STEACS subgroup.
Reperfusion and Hospital Drug Therapy Strategies According to Admission Diagnosis
| STEACS (n=788) | NSTEACS (n=1602) | Unclassified ACS (n=167) | P | |
|---|---|---|---|---|
| Reperfusion treatment, % (all/< 12 h) | — | |||
| No | 19.9/14.3 | — | — | |
| Thrombolysis | 26.8/28.8 | — | — | |
| Primary PCI | 53.3/56.8 | — | — | |
| Thrombolysis administration site, %(all/< 12 h) | — | |||
| Out-of-hospital | 30.1/33.5 | — | — | |
| Emergency room | 28.7/29.6 | — | — | |
| ICU/CCU | 41.1/33.9 | — | — | |
| Time from FMC to thrombolysis, min (all/< 12 h) | 40 [20-84]/39 [20-80] | — | — | — |
| Rescue PCI in thrombolysis, % (all/< 12 h) | 32.9/34.1 | — | — | — |
| Time from FMC to primary PCI balloon, min (all/< 12 h) | 120 [84-167]/119 [85-161] | — | — | — |
| Drug therapy on admission, % | ||||
| Acetylsalicylic acid | 98.0 | 97.6 | 95.7 | .241 |
| P2Y12 inhibitors | 97.8 | 91.2 | 83.6 | <.001 |
| Glycoprotein IIb/IIIa inhibitors | 22.3 | 4.3 | 6.1 | <.001 |
| Parenteral anticoagulants, % | 82.7 | 83.4 | 82.8 | .898 |
| Beta-blockers | 79.1 | 81.5 | 74.4 | .054 |
| Intravenous diuretics | 23.5 | 25.7 | 46.1 | <.001 |
| Inotropics/vasoactives | 11.6 | 3.2 | 7.3 | <.001 |
| Statins | 94.8 | 94.8 | 91.4 | .188 |
ACS, acute coronary syndrome; FMC: first medical contact; ICU/CCU, intensive care unit/coronary care unit; NSTEACS, non–ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEACS, ST-segment elevation acute coronary syndrome.
Unless otherwise indicated, data are expressed as median [interquartile range].
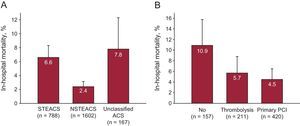
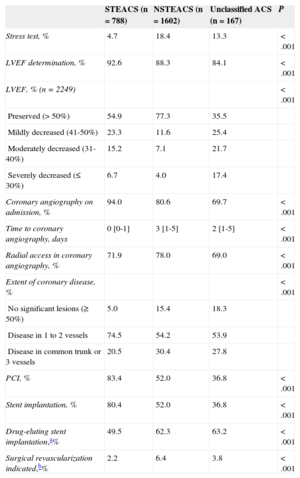
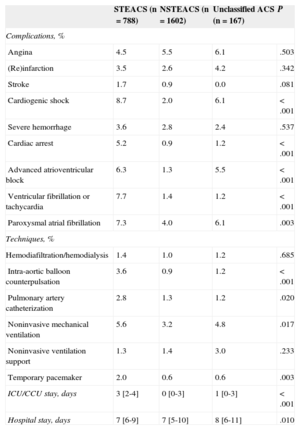
Ventricular function on admission was measured in 89% of patients, and coronary angiography was performed in 84%; radial access was most common. Revascularization with PCI was performed in 61%, and coronary surgery was indicated in 5% (Table 4). Complications were relatively rare and generally more frequent in the STEACS subgroup, and the use of special techniques was uncommon (Table 5). A total of 104 (4.1%) patients died during hospitalization. Figure 2 illustrates in-hospital mortality in the 3 subgroups, as well as in patients with STEACS according to the reperfusion treatment received.
In-hospital Examinations and Revascularization Procedures According to Admission Diagnosis
| STEACS (n=788) | NSTEACS (n=1602) | Unclassified ACS (n=167) | P | |
|---|---|---|---|---|
| Stress test, % | 4.7 | 18.4 | 13.3 | <.001 |
| LVEF determination, % | 92.6 | 88.3 | 84.1 | <.001 |
| LVEF, % (n=2249) | <.001 | |||
| Preserved (> 50%) | 54.9 | 77.3 | 35.5 | |
| Mildly decreased (41-50%) | 23.3 | 11.6 | 25.4 | |
| Moderately decreased (31-40%) | 15.2 | 7.1 | 21.7 | |
| Severely decreased (≤ 30%) | 6.7 | 4.0 | 17.4 | |
| Coronary angiography on admission, % | 94.0 | 80.6 | 69.7 | <.001 |
| Time to coronary angiography, days | 0 [0-1] | 3 [1-5] | 2 [1-5] | <.001 |
| Radial access in coronary angiography, % | 71.9 | 78.0 | 69.0 | <.001 |
| Extent of coronary disease, % | <.001 | |||
| No significant lesions (≥ 50%) | 5.0 | 15.4 | 18.3 | |
| Disease in 1 to 2 vessels | 74.5 | 54.2 | 53.9 | |
| Disease in common trunk or 3 vessels | 20.5 | 30.4 | 27.8 | |
| PCI, % | 83.4 | 52.0 | 36.8 | <.001 |
| Stent implantation, % | 80.4 | 52.0 | 36.8 | <.001 |
| Drug-eluting stent implantation,a% | 49.5 | 62.3 | 63.2 | <.001 |
| Surgical revascularization indicated,b% | 2.2 | 6.4 | 3.8 | <.001 |
ACS, acute coronary syndrome; LVEF, left ventricular ejection fraction; NSTEACS, non–ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEACS, ST-segment elevation acute coronary syndrome.
Unless otherwise indicated, data are expressed as median [interquartile range].
Complications, Special Techniques, and Duration of Hospital Stay According to Diagnosis on Admission
| STEACS (n=788) | NSTEACS (n=1602) | Unclassified ACS (n=167) | P | |
|---|---|---|---|---|
| Complications, % | ||||
| Angina | 4.5 | 5.5 | 6.1 | .503 |
| (Re)infarction | 3.5 | 2.6 | 4.2 | .342 |
| Stroke | 1.7 | 0.9 | 0.0 | .081 |
| Cardiogenic shock | 8.7 | 2.0 | 6.1 | <.001 |
| Severe hemorrhage | 3.6 | 2.8 | 2.4 | .537 |
| Cardiac arrest | 5.2 | 0.9 | 1.2 | <.001 |
| Advanced atrioventricular block | 6.3 | 1.3 | 5.5 | <.001 |
| Ventricular fibrillation or tachycardia | 7.7 | 1.4 | 1.2 | <.001 |
| Paroxysmal atrial fibrillation | 7.3 | 4.0 | 6.1 | .003 |
| Techniques, % | ||||
| Hemodiafiltration/hemodialysis | 1.4 | 1.0 | 1.2 | .685 |
| Intra-aortic balloon counterpulsation | 3.6 | 0.9 | 1.2 | <.001 |
| Pulmonary artery catheterization | 2.8 | 1.3 | 1.2 | .020 |
| Noninvasive mechanical ventilation | 5.6 | 3.2 | 4.8 | .017 |
| Noninvasive ventilation support | 1.3 | 1.4 | 3.0 | .233 |
| Temporary pacemaker | 2.0 | 0.6 | 0.6 | .003 |
| ICU/CCU stay, days | 3 [2-4] | 0 [0-3] | 1 [0-3] | <.001 |
| Hospital stay, days | 7 [6-9] | 7 [5-10] | 8 [6-11] | .010 |
ACS, acute coronary syndrome; ICU/CCU, intensive care unit/coronary care unit; NSTEACS, non–ST-segment elevation acute coronary syndrome; STEACS, ST-segment elevation acute coronary syndrome.
Unless otherwise indicated, data are expressed as median [interquartile range].
A, in-hospital mortality in all 3 patient categories. B, in-hospital mortality in the STEACS subgroup, according to reperfusion treatment received. The error bars illustrate the upper limit of the 95% confidence interval. ACS, acute coronary syndrome; NSTEACS, non–ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEACS, ST-segment elevation acute coronary syndrome.
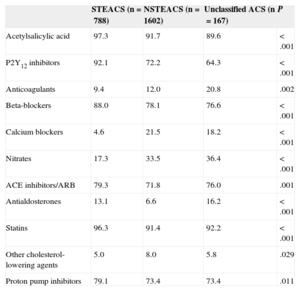
The discharge diagnosis confirmed ACS in 91.0% of patients (acute myocardial infarction in 69.2% and unstable angina in 21.8%), chest pain in 6.2%, and other diagnoses in 2.8%. In-hospital mortality according to discharge diagnosis was 5.2% in acute myocardial infarction, 0.4% in unstable angina, and 4.3% in the rest. One patient was still hospitalized at 6 months after inclusion. All other survivors were discharged home (94.8%), to another hospital (4.3%), or to another department in the same hospital (0.9%). Table 6 summarizes the treatments at discharge.
Treatment at Discharge According to Diagnosis on Admission
| STEACS (n=788) | NSTEACS (n=1602) | Unclassified ACS (n=167) | P | |
|---|---|---|---|---|
| Acetylsalicylic acid | 97.3 | 91.7 | 89.6 | <.001 |
| P2Y12 inhibitors | 92.1 | 72.2 | 64.3 | <.001 |
| Anticoagulants | 9.4 | 12.0 | 20.8 | .002 |
| Beta-blockers | 88.0 | 78.1 | 76.6 | <.001 |
| Calcium blockers | 4.6 | 21.5 | 18.2 | <.001 |
| Nitrates | 17.3 | 33.5 | 36.4 | <.001 |
| ACE inhibitors/ARB | 79.3 | 71.8 | 76.0 | .001 |
| Antialdosterones | 13.1 | 6.6 | 16.2 | <.001 |
| Statins | 96.3 | 91.4 | 92.2 | <.001 |
| Other cholesterol-lowering agents | 5.0 | 8.0 | 5.8 | .029 |
| Proton pump inhibitors | 79.1 | 73.4 | 73.4 | .011 |
ACE, angiotensin-converting enzyme; ACS, acute coronary syndrome; ARB, angiotensin receptor blockers; NSTEACS, non–ST-segment elevation acute coronary syndrome; STEACS, ST-segment elevation acute coronary syndrome.
Data are expressed as %.
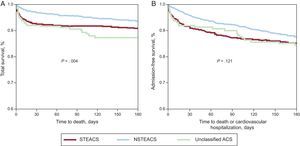
Follow-up on vital status at 6 months was available for 2381 (97.1%) of the 2452 patients discharged alive. Among those patients, mortality in that period was 3.8% (67.1% for cardiovascular cause). At 6 months, the rate of (re)infarction was 1.2% (2135 patients with the relevant data); that of stroke, 0.5% (2133 patients with data); that of readmission for cardiovascular conditions, 8.7% (2323 patients with data), and that of revascularization, 2.4% (2101 patients with data). At 30 days, mortality was 4.8% (8.0%, 3.2%, and 8.1% in the 3 diagnostic subgroups, respectively; P<.001). Figure 3 illustrates the total mortality (in-hospital and out-of-hospital) and the combined variable of mortality or rehospitalization for cardiovascular cause during the first 6 months since the index episode in the 3 subgroups.
Six-month survival curves since admission in all 3 subgroups. A, total survival. Five patients who died during this period are not included, as the date of death is unknown. B, hospitalization-free survival. ACS, acute coronary syndrome; NSTEACS, non–ST-segment elevation acute coronary syndrome; STEACS, ST-segment elevation acute coronary syndrome.
In this registry, the in-hospital mortality of patients admitted to Spanish hospitals for suspected ACS was 4.1%, a significantly lower figure than that reported in the last registry available.7 Additionally, an increase was observed in the use of recommended treatments, such as reperfusion in STEACS, coronary angiography and revascularization in NSTEACS, and secondary-prevention therapies at discharge.
Comparison With Previous StudiesUnlike other studies3,4,7 that required confirmation of ACS, our study included all patients admitted with suspected ACS. This provided information on the percentage of final diagnoses other than ACS (9% in all), but made it hard to compare between studies. However, the population characteristics are very similar to those of earlier studies, with a higher mean age than observed in PRIAMHO II (Proyecto de Registro de Infarto Agudo de Miocardio HOspitalario) and slightly lower than in the DESCARTES (Descripción del Estado de los Síndromes Coronarios Agudos en un Registro Temporal ESpañol) and MASCARA (Manejo del Síndrome Coronario Agudo. Registro Actualizado) studies, as well as a similar prevalence of diabetes mellitus and other cardiovascular risk factors and lower prevalence of previous infarction. The percentage of patients with positive markers was similar to that observed in the MASCARA registry, as was the percentage of patients in Killip class IV, although that of patients with less severe grades of heart failure on admission was lower. The inclusion of patients without confirmed ACS is unlikely to have influenced the total mortality of the study, as it was almost identical in patients in whom ACS was not finally confirmed as in the rest.
The in-hospital mortality observed in this study was significantly lower than that recorded in the MASCARA registry,7 the last analogous study conducted in Spain (4.1% vs 5.7%; P<.001). The mortality rates in the first 6 months are also lower in the 3 subgroups, although the published data7 do not allow the statistical significance of the differences between the 2 studies to be calculated.
As in the MASCARA study,7 patients with unclassified ACS were older and had a poorer risk profile and higher incidences of in-hospital and 6-month complications than the rest.
ST-segment Elevation Acute Coronary Syndrome ManagementIn total, 31% of patients had STEACS, somewhat lower than in the MASCARA registry (38%). Although this subgroup is relatively small and does not allow categorical conclusions to be drawn, several aspects are worth mentioning. First of all, the median time to first medical contact was 90min, only slightly shorter than in previous studies (96min in MASCARA). Secondly, probably in relation to the widespread use of regional STEACS protocols, most of these patients were brought to the hospital by ambulance and the rate of reperfused patients exceeded 80% (and 85% of those seen in < 12h), percentages higher than those observed in earlier studies, in which the reperfusion rate was 60% to 72%,3,4,7 and similar to those described in contemporary series in neighboring countries.11 Unlike previous studies, primary PCI was the preferred reperfusion procedure and was used approximately twice as often as thrombolysis (1:8 ratio in PRIAMHO II and 1:2 in MASCARA). As expected, mortality was higher in patients who were not reperfused than in those who were.
Some aspects of reperfusion treatment warrant a comment. The clinical practice guidelines10 recommend that thrombolysis be administered within 30min after the first medical contact. Although our study found slightly lower times than those reported in previous studies (median, 40min. vs 48min. in PRIAMHO II), they are still inadequate. Prehospital thrombolysis is recommended whenever possible,10 but was only used in a third of patients. A positive aspect is the use of rescue PCI, which was higher than that observed in the MASCARA registry7 and comparable to that of other contemporary series.12 Regarding primary PCI, the time from first medical contact to PCI was>120min (the limit allowed by the guidelines for all patients with STEACS10) in half the patients. Trending of this variable was not possible because previous studies collected door-to-balloon time.4,7 However, the data indicate that patient care should be improved by streamlining transfers to the interventional cardiology laboratory or offering thrombolysis to patients with long transfers.10
Non–ST-segment Elevation Acute Coronary Syndrome ManagementAs in the MASCARA study,7 NSTEACS was the most common presentation. The present study observed an increase in the percentage of patients who received P2Y12 receptor inhibitors while hospitalized (from 42% to 91%), parallel with a decrease in the use of glycoprotein IIb/IIIa inhibitors (from 21% to 4%, respectively). Patients with coronary angiography on admission rose from 63% to 81%, respectively, although most were not done on an urgent basis. In our study, the radial approach was frequently used for coronary angiography in all clinical presentations, which could have contributed to the relatively low incidence of complications. The PCI rate also increased compared with the MASCARA study (34% vs 52%), whereas surgical revascularization rates were similar and remained relatively low with regard to the prevalence of common trunk or triple-vessel disease. All clinical presentations showed a significant increase in the indication of acetylsalicylic acid, P2Y12 receptor inhibitors beta-blockers, renin-angiotensin system inhibitors, and statins at discharge compared with earlier studies,4,7 which may have contributed to the lower 6-month mortality observed in this study.
LimitationsThe DIOCLES registry is smaller than the latest ACS registries for Spain.4,7 Although total in-hospital mortality can be estimated with the expected confidence intervals, mortality in the various subgroups or other events cannot be estimated accurately. The main limitation of the primary endpoint was the lack of strict monitoring of the comprehensiveness of inclusions. An inclusion bias toward less serious cases could have led to an underestimation of mortality.13 For several reasons, however, we do not believe that this factor has significantly influenced the results, as sites with clearly insufficient inclusion were excluded and the proportion of excluded patients compared with the total seems appropriate and was generally homogeneous. Additionally, in-hospital mortality was similar at sites that included more patients (first quartile of the number of inclusions) than in the rest (4.5% vs 3.6%, nonsignificant differences). Lastly, it seems plausible and consistent with recent observations in neighboring countries, which have shown 30-day mortality levels in STEACS as low as 4.4%14 or 8.6%,15 that the decrease in mortality compared with that observed in the MASCARA registry is partly explained by the improvement in key health care variables, such as the percentage of patients who received reperfusion therapy or invasive management.
The proportion of patients with available follow-up for the mortality variable is high and greater than in previous studies,4,7 which minimized any potential information bias due to the fact that fewer follow-up data were available for nonsurvivors. However, the proportion of patients with information on other 6-month follow-up variables is lower (as is the case for reliability, considering that most information was obtained by phone interview), which should be considered when the results are interpreted. This may have strongly influenced the low incidence of revascularization seen in follow-up, although these data could also be explained in part by the high revascularization rate on admission and by technical advances in PCI.
CONCLUSIONSThe mortality of patients with ACS in Spain has dropped compared with the mortality reported by the last available registry, in keeping with a more frequent use of recommended treatments, such as reperfusion, revascularization, and secondary prevention measures. Several aspects, particularly time to reperfusion in STEACS, are less than optimal.
FUNDINGThis study was funded by an unrestricted grant from Daiichi-Sankyo-Lilly.
CONFLICT OF INTERESTSNone declared.
The authors acknowledge assistance from the Daiichi Sankyo/Lilly alliance to conduct this study.
Hospital Universitario de Salamanca (Javier Jiménez-Candil, Francisco Martín Herrero); Hospital Universitario Joan XXIII, Tarragona (Alfredo Bardají Ruiz, Steve Gwynn); Hospital Universitario Vall d’Hebron, Barcelona (José A. Barrabés Riu, Irene Buera Surribas); Hospital Universitario Mútua de Terrassa (Ferran Padilla Marchán); Hospital Virgen de la Concha, Zamora (José Antonio Ortiz de Murúa); Hospital de Mollet (Rosa Gilabert Gómez, Miguel Ángel Plasin Rodríguez); Complejo Hospitalario de Pontevedra (Antonio Amaro Cendón, Eva González Babarro); Hospital Universitario Puerta de Hierro, Majadahonda (Lorenzo Silva Melchor, Carlos Gutiérrez Landeluce); Hospital Universitario de Burgos (Germán Pérez Ojeda); Hospital Santiago Apóstol, Miranda de Ebro (Patricia Gil Armentia); Hospital Santa Bárbara, Soria (Valentín del Villar Sordo, Marta León Téllez); Hospital Santa Ana, Motril (Rafael Peñafiel Bukhordt, Javier Martín López); Hospital de Jove, Gijón (Alberto Riera García, Ramón-Fernando Taboada Vilariño); Hospital del Henares, Coslada (Maurice Batlle Pérez); Hospital Sant Jaume, Calella (Rafael Cuenca Luque, Manuel-Alejandro Gálvez Bobadilla); Hospital Universitario Virgen de la Macarena, Sevilla (Juan Carlos García Rubira, Rafael Hidalgo Urbano); Hospital General de Segovia (Pablo Ancillo García, José Joaquín Cortina Gómez); Hospital Universitario Marqués de Valdecilla, Santander (Natalia Royuela Martínez, Ángela Canteli Álvarez); Hospital Clínic, Barcelona (Magda Heras, Jaime Hernández Ojeda); Hospital Universitario Virgen de las Nieves, Granada (Rafael Melgares Moreno); Hospital Universitario Donostia, Donostia-San Sebastián (Francisco de la Cuesta Arzamendi); Hospital Infanta Cristina, Badajoz (Antonio Merchán Herrera, Luis-Javier Doncel Vecino); Hospital de Mérida (Domingo Marzal Martín, Luis Salvador Ramos); Hospital Universitario Severo Ochoa, Leganés (Frutos del Nogal Sáez); Hospital Clínico San Cecilio, Granada (Jesús Gabriel Sánchez Ramos); Hospital San Agustín, Avilés (Victor Manuel Rodríguez Blanco); Hospital Moisès Broggi, Sant Joan Despí (Roman Freixa Pamias, Antoni Carol Ruiz); Hospital Clínico San Carlos, Madrid (Antonio Fernández-Ortiz, Hernán Mejía Rentería); Complejo Hospitalario de Navarra, Pamplona (Nuria Basterra Sola, Javier Martínez Basterra); Hospital de la Serranía, Ronda (Luis Miguel Tamargo Gutiérrez, Aurora Ruz Zafra); Hospital General Juan Cardona, Ferrol (Francisco Javier Roselló Llerena); Hospital Regional Universitario Carlos Haya, Málaga (Manuel de Mora Martín, Ana García Bellón); Hospital Universitario Río Hortega, Valladolid (M. Jesús Rollán Gómez, Cristina Tapia Ballesteros); Hospital Galdakao, Usansolo (Iñaki Lekuona Goya, Germán Zugazabeitia Irazábal); Hospital General Universitario Los Arcos del Mar Menor, San Javier (José Antonio Giner Caro); Hospital de Jerez, Jerez de la Frontera (Santiago Jesús Camacho Freire, Javier León Jiménez); Hospital Universitario Puerta del Mar, Cádiz (Rafael Vázquez García, Daniel Bartolomé Mateo); Hospital Arnau de Vilanova, Valencia (Jorge Ruvira Durante, M Asunción Hervás Botella); Hospital Virgen de los Lirios, Alcoy (Elvira Marco Francés, Guillermo Grau Jornet); Hospital Clínico Universitario de Valencia (Vicente Bodí Peris, Ernesto Valero Picher); Hospital General San Jorge, Huesca (Clara Bergua Martínez); Hospital La Inmaculada, Huércal-Overa (Andrés May Garayalde); Hospital de Poniente, El Ejido (Jacinto Benítez Gil); Hospital General Universitario Reina Sofía, Murcia (Tomás Vicente Vera, Francisco Felices Abad)