Oxygen saturation by pulse oximetry is commonly used for monitoring critical patients, but its utility as a diagnostic marker of acute heart failure has not been assessed. This study analyzed the diagnostic role of oxygen saturation by pulse oximetry in a series of patients with acute myocardial infarction.
MethodsIn a prospective observational cohort study of 220 consecutive patients with acute myocardial infarction, data collection included baseline oxygen saturation by pulse oximetry (without oxygen), physiologic measurements, Killip class and data from portable chest radiography, recorded at the same hour on each of the first three days after admission. Patients were followed up for one year.
ResultsThere were 612 assessments. Baseline oxygen saturation by pulse oximetry decreased progressively in relation to the presence and the severity of acute heart failure assessed by Killip classes 1 to 3 (mean: 95, 92 and 85, respectively; P<.001) or by radiology score 0 to 4 (95, 94, 92, 89 and 83, respectively; P<.001), with a correlation coefficient of 0.66 and 0.63, respectively. Receiver operating characteristic curves disclosed the cut-off of oxygen saturation by pulse oximetry<93 to have the greatest area, with a sensitivity of 65%, specificity 90%, and overall test accuracy 83%. Patients grouped according to lowest oxygen saturation by pulse oximetry showed significantly different rates of one-year mortality or rehospitalization for heart failure.
ConclusionsBaseline oxygen saturation by pulse oximetry is useful in establishing the diagnosis and severity of heart failure in acute settings such as myocardial infarction and may have prognostic implications.The diagnosis may be suspected when baseline oxygen saturation by pulse oximetry is <93.
Keywords
.
IntroductionAcute heart failure (AHF) is a frequent complication in patients with acute myocardial infarction (AMI).1, 2 The incidence may be rather variable depending on the diagnostic criteria.3 In clinical practice, diagnosis is initially made on admission by anamnesis and physical examination.4 However, identification of this complication is frequently difficult and it is necessary to obtain complementary data from portable chest radiography5 or other techniques such as echocardiography,6 pulmonary wedge catheter,7 or biological markers.8 Because AHF affects pulmonary function,9 gas exchange could be altered even in mild forms. Although AHF has been associated with hypoxemia,10 the diagnostic contribution of oxygen saturation by bedside pulse oximetry (SpO2) has not been analyzed. Most of the previous studies using this technique in AMI were limited to investigating the incidence of hypoxemia or nocturnal sleep apnea syndrome.11, 12, 13, 14, 15 Therefore, we conducted an observational study to assess the value of baseline SpO2 for the diagnosis and severity of AHF in patients with AMI, a homogeneous population with a high prevalence of this complication.
Methods Patient PopulationOur institutional ethics committee approved the study design and all patients gave their informed consent. All consecutive patients of any age with ST and non-ST segment elevation AMI admitted to our critical/semi-critical intensive care unit were considered for inclusion. AMI was defined by prolonged chest pain with ischemic electrocardiographic changes and elevated myocardial necrosis biomarkers. Patients were included irrespective of the treatment received prior to admission (primary coronary angioplasty, fibrinolytic or conventional treatment). Patients with moderate to severe chronic obstructive pulmonary disease (GOLD classes II-III) and those who developed cardiogenic shock were excluded.
Study DesignThis was a prospective observational cohort study. Patients were continuously monitored by pulse oximetry during the first three days, using modular Hewlett-Packard (SpO2/Plet M1020A) pulse oximeters with a central station monitor. The target SpO2 was “morning baseline SpO2” (MB-SpO2), which was registered by nurses in awake patients on the morning round, usually after chest radiography, and taking the most stable value on room air. In patients receiving oxygen therapy it was recorded when a steady value was reached, after several minutes of oxygen discontinuation.
Physical examination data was recorded by staff physicians (Killip class)4 on admission and daily morning rounds thereafter. Portable chest radiographs were also obtained daily during rounds and were evaluated using a radiology score by consensus of 2 observers and sometimes 3 in case of initial disagreement. This score was based on Battler et al. classification,5 establishing 5 categories: (0) normal; (1) vascular redistribution to upper lung fields; (2) interstitial edema defined by B-Kerley lines or prominent and diffuse hiliar contour with vascular redistribution; (3) localized edema with alveolar infiltrates in less than 50% of the lung fields, mainly hilo-basal, and (4) diffuse alveolar edema with alveolar infiltrates in 3 or 4 quadrants.
Data obtained on the first morning after admission was introduced as “first day”. Thus, the interval from the onset of AMI to that moment could be variable. However, data on the following days were also collected in the morning but at constant intervals up to 3 days. Oxygen therapy was administered if baseline SpO2 was lower than 93%. Cardiac necrosis markers (MB fraction creatine kinase/troponin T) were obtained on admission and every 6h, until peak value was identified. Natriuretic peptides were not available in our center at the time of study recruitment.
The variables registered during daily rounds were heart rate, respiratory rate, blood pressure, MB-SpO2, Killip class, and radiology score. The variable “lowest baseline SpO2” (LB-SpO2) was obtained retrospectively upon discharge from the intensive care unit (ICU), taking the lowest SpO2 value among those recorded during the whole acute cardiac care stay.
Ventricular function was assessed by echocardiography, which was performed during hospitalization. After discharge, patients were followed up for one year and death or rehospitalization with AHF was registered.
Diagnosis of Acute Heart FailurePatients with Killip classes 2 or 3 were initially considered to have AHF in the Bayesian analysis. However, an abnormal chest radiography was required to confirm this diagnosis in such a way that patients who presented normal radiology (score 0) were considered not to have AHF (Killip false positive) regardless of the assigned Killip class. Conversely, patients initially evaluated as Killip 1 on physical examination who presented unequivocal signs of pulmonary congestion on radiology (score 3-4) were considered to have AHF (Killip false negative).
Statistical AnalysisThe total data registered during the 3 days of monitoring accounted for more than 600 determinations for every one of the 6 study variables (Killip class, radiology score, MB-SpO2, heart rate, respiratory rate and blood pressure). Receiver operating characteristic (ROC) curves from these simultaneous determinations were used to analyze the test accuracy for the diagnosis of AHF for different cut-off levels of MB-SpO2, heart, and respiratory rates.
When patients were analyzed, their LB-SpO2 was individually assigned. Comparisons between means were analyzed using the Student t test or analysis of variance (ANOVA). In variables with no normal distribution or no homogeneous variances, a nonparametric test (Kruskal-Wallis or Mann-Whitney) was used. Pair-wise multiple comparisons were performed by least-significant differences or Tamhane test when variances were not equal. Noncontinuous variables were analyzed by Fisher exact test. Continuous variables were reported as mean (standard deviation). All P values were for two-tailed comparisons.
Independent factors were analyzed by logistic regression analysis. Kaplan-Meier event-free (death or rehospitalization with AHF) survival analysis was used for the follow-up evaluation, assigning patients to one of three groups, according to their LB-SpO2. The range of LB-SpO2 for these groups was guided by the 95% confidence intervals (95%CI) of the mean SpO2 in each Killip class group. Consequently, these groups were: group 1 (SpO2>93%); group 2 (SpO2=90%-93%), and group 3 (SpO2<90%).
ResultsOver a 2-year period, 220 consecutive patients with AMI, with and without ST segment elevation, were admitted to our intensive/semi-intensive care unit and accepted for participation in the study. The study was approved by our Ethics and Research Committee and was carried out in the year 2000. Fourteen patients (6%) developed cardiogenic shock or needed mechanical ventilation (invasive or noninvasive) and were subsequently excluded. In the remaining patients there were 122 with ST-segment elevation and 9 with left bundle branch block. Non-Q wave AMI was the final diagnosis in 106 patients.
The characteristics of the patients based on the incidence of AHF are shown in Table 1. Overall, patients with AHF were older, less predominantly men, with higher rates of previous hypertension, diabetes, myocardial infarction and heart failure than patients without AHF.
Table 1. Characteristics of 206 Patients With Acute Myocardial Infarction According to the Presentation of Acute Heart Failure
| No AHF (n = 133) | AHF (n = 73) | P | |
| Characteristics | |||
| Age, years | 61.5±12.4 | 70±9.4 | <.001 |
| Male | 105 (79) | 47 (64) | .031 |
| Diabetes | 34 (26) | 35 (48) | .002 |
| Hypertension | 59 (45) | 48 (69) | .002 |
| Prior myocardial infarction | 19 (15) | 28 (39) | <.001 |
| Prior heart failure | 6 (5) | 22 (31) | <.001 |
| LBBB | 1 (0.8) | 8 (11) | .001 |
| ST-Segment elevation | 84 (63) | 48 (66) | .760 |
| Outcomes | |||
| 30-day mortality | 0 | 9 (12.3) | <.001 |
| One-year mortality | 6 (4.9) | 18 (26.1) | <.001 |
| Physiologic measurements | |||
| Lowest SpO2 | 93.4±2.1 | 87.9±6.3 | <.001 |
| Systolic blood pressure, * mmHg | 118.6±14.6 | 118.9±17.5 | .880 |
| Heart rate, * bpm | 71.7±11.7 | 81.1±13.5 | <.001 |
| Respiratory rate, * breaths/min | 18.9±2.4 | 21.5±3.8 | <.001 |
| LVEF, % | 60.1±8.7 | 45.8±10.5 | <.001 |
| End-diastolic diameter, mm | 52.3±5.7 | 54.7±5.9 | .010 |
AHF, acute heart failure; LBBB, left bundle branch block; LVEF, left ventricle ejection fraction; SpO2, oxygen saturation by pulse oximetry.
Data are expressed as mean ± standard deviation or no. (%).
* Average values obtained on the 3 days of the study during the morning rounds.
After 3 days the cumulative incidence of some degree of AHF was 35%. Two thirds of the patients presented AHF on admission whereas the rest of the patients developed this complication thereafter. Regarding symptoms, AHF was clinically silent in the majority of the patients and less than 20% showed some degree of shortness of breath. Mortality after 30 days and at 1 year was significantly greater in patients with AHF.
Physiologic measurements are also presented in Table 1. Patients with AHF showed significantly higher respiratory and heart rates and lower LB-SpO2 and left ventricular ejection fraction, while there were no differences in blood pressure.
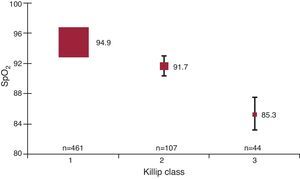
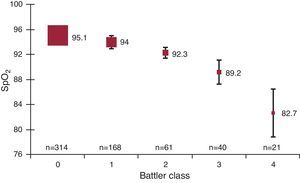
Distribution of mean MB-SpO2 values in relation to the degree of AHF is shown in Figure 1, Figure 2. Median MB-SpO2 was significantly lower according to the degree of AHF measured either by Killip classes (n=612) or Battler scores (n=604). All intergroup pair- wise comparisons were also significantly different. There were also significant differences between respiratory rates for Killip classes 1 to 3 (19 [3], 21 [4], and 26 [8], respectively; P<.001) and for Battler scores 0 to 4 (19 [3], 20 [3], 21 [5], 24 [7], and 26 [7], respectively; P<.001). All intergroup pair-wise comparisons for respiratory rate were also significantly different.
Figure 1. Baseline oxygen saturation by pulse oximetry values in relation to the degree of heart failure assessed by physical examination (Killip classes) for all measurements. Squares are means; bars (standard deviation). SpO2, oxygen saturation by pulse oximetry.
Figure 2. Baseline oxygen saturation by pulse oximetry values in relation to the degree of heart failure assessed by radiology score (Battler classes) for all measurements. Squares are means; bars (standard deviation). SpO2, oxygen saturation by pulse oximetry.
Diagnostic AccuracyRegarding measurements (n=612), the correlation coefficients between MB-SpO2 and Killip class and Battler score were −0.666 and −0.619, respectively (P<.001). Regarding patients, the correlation coefficient between LB-SpO2 and left ventricular ejection fraction was 0.433; P <0.001.
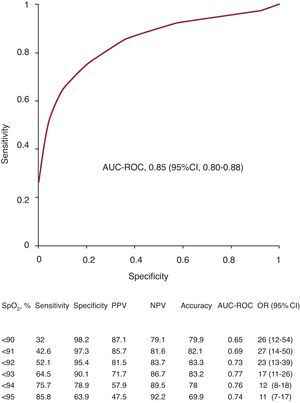
The MB-SpO2 measurements were plotted in a ROC curve for the diagnosis of AHF (Figure 3). The cut-off level of SpO2<93 showed the greatest area under the curve with the highest test accuracy (83%). The cut-off value for patients with chronic bronchitis (n=45) with greater area was SpO2<93 as well. The ROC curves were also plotted for respiratory and heart rate. The cut-off with greater area for respiratory rate was >21 breaths/min (area, 0.64), with a test accuracy of 59% (odds ratio=2.19; 95%CI, 1.53-3.16). The cut-off value for heart rate was >76 bpm (area, 0.61) and the test accuracy 73% (odds ratio=4.21; 95%CI, 2.8-6.3).
Figure 3. Receiving operating characteristics curve for oxygen saturation by pulse oximetry and acute heart failure. A table with information about different cut-off points is attached. 95%CI, 95% confidence interval; AUC, area under curve; NPV, negative predictive value; OR, odds ratio; ROC: receiver operating characteristic; SpO2, oxygen saturation by pulse oximetry; PPV, positive predictive value.
There were 29 patients who were misclassified by Killip score, 20 (10%) false negatives and 9 (4%) false positives. The combination of MB-SpO2<93 and Killip classes (≥2) or respiratory rate (>21 breaths/min) increased the specificity to 99.4% and 97% and the positive predictive value to 97.2% and 78%, respectively.
Follow-upMean follow-up was 13 months and was completed in 192 patients (93%). Multivariate logistic regression analysis showed age and AHF to be the independent predictive variables for 1-year death or rehospitalization for AHF (Table 2). LB-SpO2 was only an independent factor when AHF was not introduced in the analysis.
Table 2. Risk Factors for One-year Death or Rehospitalization for Heart Failure
| Univariate analysis | Multivariate analysis | |||
| OR (95%CI) | P | OR (95%CI) | P | |
| Acute heart failure | 7.8 (3.6-17.1) | <.001 | 7.1 (2.7-19) | <.001 |
| Measurements | ||||
| SpO2 a | 5.5 (2.6-11.8) | <.001 | ||
| Left ventricular function b | 4.3 (1.9-9.6) | .001 | ||
| Heart rate b | 2.5 (1.2-5) | .013 | ||
| Respiratory rate b | 2.5 (1.2-5) | .02 | ||
| Clinical characteristics | ||||
| History of heart failure | 5.5 (2.3-12.9) | <.001 | ||
| Prior myocardial infarction | 3.5 (1.7-7.4) | .001 | ||
| Diabetes | 2.5 (1.2-5) | .015 | ||
| Hypertension | 2.2 (1.05-4.6) | .049 | ||
| Age c | 1.13 (1.07-1.18) | <.001 | 1.12 (1.05-1.18) | <.001 |
95%CI, 95% confidence interval; OR, odds ratio; SpO2, oxygen saturation by pulse oximetry.
The OR reflects the odds for patients with the characteristic in question, as compared to those without the characteristic.
a SpO2 is a categorical variable (cut-off <93) obtained by taking the lowest value registered on the rounds for each patient.
b Categorical variables (cut-off: ejection fraction <0.50; heart rate >76 bpm; respiratory rate >21 breaths/min).
c The OR for age represents the exponent for each year of age in the logistic equation.
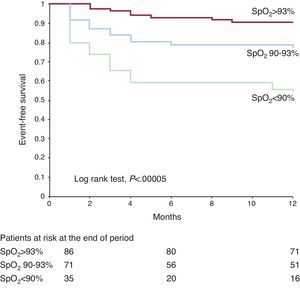
Kaplan-Meier event-free (mortality and rehospitalization for AHF) survival curves for the 3 LB-SpO2 groups are shown in Figure 4. There were significant differences in the event-free rates between the 3 groups as well as in pair-wise group comparisons: group 1 vs group 2 (90.7% vs 76.1%; P=.01), group 2 vs group 3 (76.1 vs 54.3%; P=.01) and group 1 vs group 3 (90.7 vs 54.3%; log rank test, P=.00005).
Figure 4. Kaplan-Meier event-free survival curves for three groups of oxygen saturation by pulse oximetry. Events are death or rehospitalization with heart failure within the first year. Numbers are patients at risk in each group after 1, 6, and 12 months. SpO2, oxygen saturation by pulse oximetry.
DiscussionThis study shows the utility of baseline SpO2 as a complementary tool to establish the diagnosis and severity of AHF. We chose to perform the study in patients with AMI because of the high prevalence of AHF in this population. The main advantage of SpO2 lies in the fact that is noninvasive, it can be monitored continuously, and it is not affected by interobserver or intraobserver variability. The finding of a baseline SpO2 lower than 93 may be considered a signal of AHF and a warning to clinicians about this complication which may be especially useful between rounds. The lower the SpO2 value, the higher the probability and severity of AHF.
The results also stress the role of measuring respiratory rate and heart rate. These findings are relevant because respiratory rate is not usually considered a diagnostic marker of AHF. Although heart rate is commonly assessed for the diagnosis, it can be altered by the use of beta-blockers. The association of a decreasing SpO2 with an increasing respiratory rate may reinforce the suspicion of AHF in patients with doubtful diagnostic criteria.
Our study found good correlations between baseline SpO2 and the degree of AHF or left ventricular ejection fraction. These findings were in accordance with previous trials analyzing oxygen saturation by CO-oximetry from arterial blood samples, which showed a close correlation between the defects in gas exchange and the severity of AHF or the pulmonary capillary wedge pressures,10, 16, 17, 18 although this correlation decreased in chronic patients.19 Hypoxemia in AHF is explained by an increase in veno-arterial shunting and ventilation-perfusion imbalance.10, 16, 17, 18 Despite this well-known interaction, however, the determination of oxygen saturation (SaO2) by arterial puncture as a diagnostic marker of AHF is not a common practice and this valuable information is frequently missed. Although SpO2 values are closely related to SaO2 obtained by CO-oximetry, the accuracy of pulse oximetry may decrease in special situations such as severe hypoxemia, anemia, acidosis, pigmentation, low perfusion states, and the use of vasoactive drugs.20, 21 Therefore, SpO2 and SaO2 are not totally equivalent and the information in the literature about the role of SpO2 in AHF is scarce. Some authors have reported their experience with SpO2 in detecting Cheyne-Stokes respiration in patients with heart failure22 but, to our knowledge, no previous study has tested the utility of SpO2 for the diagnosis of AHF.
The diagnosis of AHF may be difficult in patients with AMI because it is often silent.23 In our series, the majority of patients with AHF did not show shortness of breath. Therefore, because AHF may be misdiagnosed in many cases by physical examination24, 25 (5%-10% of patients in our study were misclassified), the diagnostic criteria of AHF in this study required abnormal radiology because this technique enhances the sensitivity and diagnostic accuracy.3, 23 Chest radiography is considered a class I recommendation in the European Society of Cardiology guidelines on the diagnosis and treatment of AHF.26, 27 In these guidelines, SpO2 also received the same consideration for monitoring, not for diagnosis.
In recent decades the evaluation of ventricular function and filling pressures by echocardiography has become a widespread practice in the assessment of AHF, often replacing invasive approaches like pulmonary catheter.27, 28, 29 Nevertheless, the information provided by echocardiography is not continuous. Biochemical markers such as natriuretic peptides are frequently abnormally elevated on admission30 and have better prognostic than diagnostic value in patients with AMI.8 In this context, SpO2 may become a complementary, continuous, and low-cost parameter for assessing AHF with prognostic implications, as shown in the follow-up.
LimitationsThis study had some limitations, including the lack of determination of natriuretic peptides, which were not commonly used in our institution when the study was performed, and the absence of a gold standard for the diagnosis of AHF in patients with AMI. Other limitations were the registry format that introduced data from each patient several times, the inability to achieve a completely blinded clinical assessment, and finally, the fact that patients with AHF were older and age might produce a decrease in SpO2 by itself. Furthermore, we assessed the merits of SpO2 as a diagnostic marker of AHF in patients with AMI, but this could not be tested in patients with significant chronic obstructive pulmonary disease or shock and it should be evaluated in the future in other acute care conditions. Finally, this series should not be considered as representative of the incidence of AHF in patients with acute coronary syndromes, as our population was recruited in a single community hospital with strongly biased admissions.
ConclusionsIn this study, baseline SpO2 provided reliable information for establishing the diagnosis and severity of AHF as a complication of AMI with a warning cut-off value<93. The use of pulse oximetry for diagnosis purposes may be recommended when managing patients at risk of AHF.
FundingOur own institution financed the study.
Conflicts of InterestNone declared.
Acknowledgments
We thank physicians Gaietà Permanyer, Xavier Bosch, Pilar Tornos, Jordi Otero, and Pere Codinach for their critical review of the paper; physicians Rosario Cañizares, Jaume Padró, and Josep Ballús, as well as the entire nursing team of our intensive care unit, for their technical support in collecting data; the epidemiologist Montserrat Martin for statistical support; Mitsi Ito and Joshua Morris for help with language in writing the paper. All of them gave their consent to be mentioned in this section.
Received 12 February 2012
Accepted 18 February 2012
Corresponding author: Unidad de Cuidados Intensivos, Hospital Sant Joan Despí Moisès Broggi, Consorci Sanitari Integral, Universidad de Barcelona, Jacint Verdaguer 90, 08970 Sant Joan Despí, Barcelona, Spain. jmasip@ub.edu