Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary syndrome. Most patients are empirically treated with beta-blockers and antiplatelet drugs. The Beta-blockers and Antiplatelet agents in patients with Spontaneous Coronary Artery Dissection (BA-SCAD) is an academic, pragmatic, prospective, randomized, open-label, blinded-endpoint clinical trial, performed under the auspices of the Spanish Society of Cardiology, to assess the efficacy of pharmacological therapy in patients with SCAD.
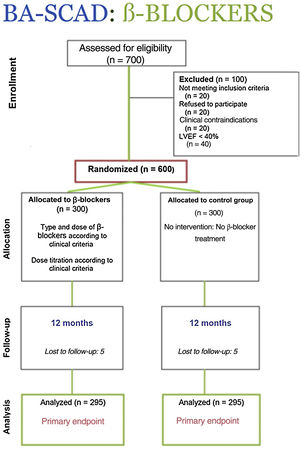
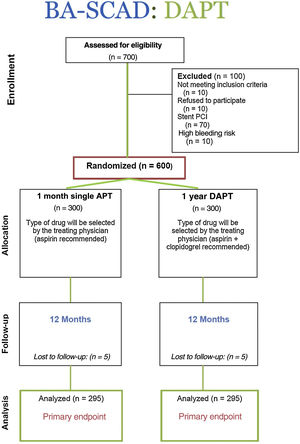
MethodsUsing a 2 x 2 factorial design, 600 patients will be randomized (1:1/1:1) to: a) beta-blockers (yes/no) and b) “short” (1 month) vs “prolonged” (12 months) antiplatelet therapy. Only patients with preserved left ventricular ejection fraction will be randomized to beta-blockers (yes/no) because patients with reduced left ventricular ejection fraction will receive beta-blockers according to current guidelines. Similarly, only conservatively managed patients (ie, no coronary intervention) will be randomized to the antiplatelet stratum, as patients requiring coronary interventions will receive 1-year dual antiplatelet therapy. The primary efficacy endpoint includes a composite of death, myocardial infarction, stroke, coronary revascularization, recurrent SCAD, and unplanned hospitalization for acute coronary syndrome or heart failure at 1 year. The primary safety endpoint will be bleeding. All patients will be clinically followed up yearly. A comprehensive set of additional substudies (clinical, imaging, revascularization, biomarkers, inflammatory, immunologic, pharmacogenetics, and genetic) will be conducted to ensure a holistic view of this unique and challenging clinical entity.
ConclusionsThe results of the BA-SCAD randomized clinical trial will advance our knowledge in the treatment of patients with SCAD.
The study was registered at ClinicalTrials.gov (Identifier: NCT04850417).
Keywords
Spontaneous coronary artery dissection (SCAD) is a relatively rare cause of acute coronary syndrome (ACS).1–8 However, SCAD accounts for up to 25% of acute myocardial infarctions (MI) in young to middle-aged women.1,2 The increasing number of patients currently being diagnosed with SCAD can be explained by enhanced clinical awareness, recognition of the different angiographic presentation patterns, and wide use of intracoronary imaging (optical coherence tomography or intravascular ultrasound) to confirm the diagnosis, together with the widespread use of early coronary angiography in ACS patients.1–8 Our understanding of the disease has significantly advanced in the last few decades since its initial description, but most information has been gathered during recent years. Initially, the available information was largely inconsistent, coming from a myriad of case reports and small retrospective observational series. Recent data on common genetic variants has shown that the clinical association between SCAD and other vascular disorders (eg, fibromuscular dysplasia, cervical, and intracerebral dissection, and migraine) is mirrored by elements of shared genetic risk.1,2,9 Whenever possible, a conservative initial medical management is advocated in these patients, not only because the prognosis is usually favorable following clinical stabilization after the initial ischemic insult, but also because spontaneous healing of the coronary wall is part of the natural history of this clinical entity.1,2,10,11 Moreover, results of revascularization in this setting tend to be suboptimal.11–13 Recently, large registries, including some prospective nationwide studies, have significantly enriched our knowledge of SCAD.5–8,13–16 However, evidence on the value of existing medical therapies in patients with SCAD is very limited and is based on observational data only. In particular, the value of the most frequently used medical therapies, namely beta-blockers and antiplatelet agents, remains unsettled.1,2
In this prospective, multicenter, pragmatic, randomized clinical trial, we will assess the efficacy and safety of beta-blockers and antiplatelet agents in patients with SCAD.
METHODSThe Beta-blockers and Antiplatelet agents in patients with Spontaneous Coronary Artery Dissection (BA-SCAD) is a prospective, multicenter, randomized clinical trial that will assess the clinical value of beta-blockers and antiplatelet drugs in patients with SCAD. The study has been registered (ClinicalTrials.gov Identifier: NCT04850417) and has been approved by the Ethics Committee of the Coordinating Center (Hospital Universitario de La Princesa) and the Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). The trial is sponsored and promoted by the Spanish Society of Cardiology and will be managed by the Research Agency of the Spanish Society of Cardiology.
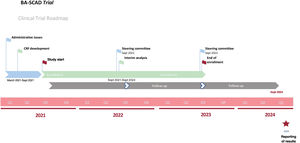
The angiographic diagnosis of SCAD includes the classic double lumen morphology and the characteristic angiographic images of intramural hematoma as defined in recent consensus documents.1,2 Systematic use of intracoronary imaging is not required to establish the diagnosis.1,2 Eligible patients meeting all inclusion and no exclusion criteria (table 1) will be enrolled (ideally within 48hours of diagnosis) after providing signed informed consent. Through a factorial (2 x 2) design, patients will be centrally randomized using a computer-generated code. When required by the local investigator, diagnostic angiograms will be immediately reviewed online (at the time the patient is considered for inclusion) by an expert on SCAD. Randomization will be performed in a blinded manner with the use of a computerized algorithm. The randomization system will be readily accessible online 24/7/365. Figure 1 displays the suggested timeframe of the BA-SCAD trial.
Inclusion and exclusion criteria
| Inclusion criteria |
| Diagnosis of SCAD on coronary angiography during the index hospitalizationa |
| Patients admitted for acute coronary syndrome or any other manifestation of myocardial ischemia |
| Written informed consent |
| “General” exclusion criteria |
| Cardiogenic shock or severe hemodynamic instability |
| Concomitant severe heart disease requiring surgical correction (in <2 years) |
| Any major medical condition seriously limiting life expectancy (< 2 years) |
| Pregnancy |
| Participation in another clinical trial |
| “Partial” exclusion criteria |
| For only 1 treatment stratum (ie, exclusions affecting only 1 of the 2 treatment strategies; the patient should be randomized in the other stratum of the trial) |
| LVEF <40% as detected by any imaging technique during hospitalizationb |
| Known allergy, intolerance, or absolute contraindication to beta-blockersb |
| Known allergy, intolerance, or absolute contraindication to antiplatelet drugs (note that different antiplatelet agents may be used as required when a particular agent cannot be used)c |
| Clinical need for oral anticoagulationc |
| Coronary revascularization before randomizationc |
LVEF, left ventricular ejection fraction; SCAD, spontaneous coronary artery dissection.
When required by the local investigator, angiographic images will be reviewed online by a SCAD expert at the angiographic core lab to confirm the diagnosis of SCAD before randomization. Intracoronary imaging may be needed in selected cases to confirm diagnosis.
For the main study, only data available during routine clinical practice will be prospectivelly collected and there will be no additional tests mandated by the protocol diverging from the standard of care. This is a low-intervention trial that uses medicinal products as authorized, and the additional diagnostic or monitoring procedures represent no added risk compared with routine clinical practice.
PRECIS (PRagmatic Explanatory Continuum Indicator Summary) is the acronym for a tool developed to identify where the trial is located within a pragmatic/explanatory continuum. The PRECIS-2 assessment of pragmatic trials was evaluated for the design of the current study. Nine domains were assessed (scores 0-5, considering a score of 5 identical to routine usual care). The following scores were obtained: eligibility (5/5), recruitment (5/5), setting (5/5), organization (3/5), flexibility delivery (4/5), flexibility (adherence) (3/5), follow-up (3/5), primary outcome (5/5), primary analysis (5/5).
As in most pragmatic trials, the randomly assigned groups will not be masked. Therefore, efforts will be made to minimize potential biases related to the reporting of major events, including the individual clinical endpoints assessed in this trial. Therefore, this approach will be selected following the methodological recommendations suggested by the Prospective Randomized Open-label Blinded-Endpoint (PROBE) initiative.
MedicationsThis is a pragmatic study where medications, during hospitalization and at follow-up, will be re prescribed by the treating physician.1,2
- •
Beta-blocker therapy will be started on the day of randomization. The type and dose of beta-blocker, including titration of therapy to ensure an adequate effect, will be at the discretion of the treating physician (the comparator arm will receive no beta-blocker therapy).
- •
Short (1-month) vs prolonged (12-month) antiplatelet therapy will be initiated on the day of randomization. Aspirin monotherapy for 1 month is the recommended regimen in the short antiplatelet arm. However, investigators will be given the option to consider the use of dual antiplatelet therapy (DAPT) for a period no longer than 1 month. This approach has been implemented given that DAPT remains an integral part of conservatively managed ACS patients.3,4 Alternatively, for patients randomized to prolonged DAPT, the use of both aspirin and clopidogrel for 1 year is recommended. Although the potent P2Y12 inhibitors (ie, ticagrelor and prasugrel) are preferred over clopidogrel in ACS patients according to practice guidelines,3,4 there are no data for their use in SCAD.1,2 Nevertheless, the pragmatic nature of this trial will allow the choice of P2Y12 inhibitor to be at the discretion of the treating physician and at a dosing regimen recommended by guidelines and in line with the product label. Patients allocated to the prolonged antiplatelet regimen arm will be recommended to maintain a single antiplatelet agent (preferably aspirin) after the first year.
Other cardiovascular medications will be prescribed at the discretion of the treating physician according to guideline recommendations.3,4 Anticoagulation will be discontinued once SCAD has been confirmed on angiography. Since patients with reduced left ventricular ejection fraction (LVEF) <40% have a class IA recommendation for receiving beta-blockers and additional medications (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) according to guidelines,3 these patients will not be randomized in the stratum of beta-blockers but only in the antiplatelet part of the study. Similarly, patients requiring coronary revascularization will be treated with prolonged DAPT and, therefore, will not be included in the antiplatelet part of the trial. Patients with an indication for oral anticoagulants (eg, atrial fibrillation, mechanical valves), will not be randomized to the antiplatelet stratum of the trial (table 1). Coronary risk factors will be aggressively treated and followed up as recommended by clinical practice guidelines.3,4 Antihypertensive agents different from beta-blockers should be prescribed as required in patients not allocated to beta-blockers, according to the guidelines. Women of child-bearing age will be advised to use a highly effective contraceptive method considering the pregnancy-associated risk of recurrent SCAD. Cardiac rehabilitation will be recommended.1,2
Importantly, chronic obstructive pulmonary artery disease (COPD) will not be considered a contraindication to the use of beta-blockers. A recent large meta-analysis17 demonstrated that the use of beta-blockers in cardiac patients with COPD is not only safe but reduces their mortality. Cardioselective beta-blockers did not affect the action of bronchodilators and actually reduced COPD exacerbations.17 The use of cardioselective beta-blockers will be recommended in patients with COPD allocated to this therapeutic strategy.
EndpointsThe primary endpoint of the study is the composite of death, MI, stroke, coronary revascularization, and recurrent SCAD or hospital admission for ACS-with dynamic electrocardiographic changes-or heart failure, at 1-year follow-up. For a suspected ACS episode to qualify as an event, dynamic electrocardiographic changes, or abnormal cardiac markers (ie, MI) are required. This was predefined to avoid the inclusion of episodes of nonischemic chest pain that are frequently seen in these patients. The 2 x 2 factorial design of the BA-SCAD trial enables 2 study hypotheses to be addressed. The first study hypothesis is that treatment with beta-blockers will reduce the primary endpoint compared with no treatment with beta-blockers. The second study hypothesis is that short (1-month) single antiplatelet therapy will be noninferior to 1-year DAPT on the primary endpoint. However, prolonged DAPT will cause an increased risk of bleeding (secondary endpoint) as assessed using the Bleeding Academic Research Consortium (BARC) criteria. Specifically, both BARC ≥ 2 and ≥ 3 will be considered for the safety endpoint. However, BARC ≥ 2 bleeding is a particularly clinically relevant problem for these relatively young patients.
A secondary combined clinical endpoint (composite of death, MI, stroke, coronary revascularization, recurrent dissection, hospital admission for ACS or heart failure admission, and bleeding) will be used to assess the “net clinical benefit” of the antiplatelet strategy. Individual components of the primary endpoint will be assessed as secondary endpoints. Moreover, major secondary combined endpoints will be the composite outcome measure of “hard” clinical events (including death, MI, stroke, recurrent dissection, and coronary revascularization) and also combined death and MI alone. The primary endpoint, all the combined secondary endpoints and the individual events will be also assessed at an extended 2-year clinical follow-up.
The current study design is underpowered for a final combined “hard” endpoint (death, MI), but following the recommendations of the International Scientific Committee, this important outcome measure should be prespecified to allow the possibility of prospective integration of the current study with co-organized international initiatives that may be devised to complement the current trial. Members of the International Scientific Committee are committed to use the study protocol to develop research initiatives to obtain additional grant support in their respective countries (UK-ESC, Canada, USA).
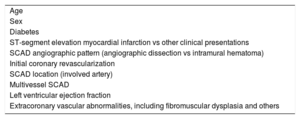
The main objective of the trial is to evaluate the clinical efficacy of the 2 most widely used pharmacological therapeutic strategies in patients with SCAD in clinical practice. Namely, to assess the role of beta-blockers and different antiplatelet regimens (short [1 month] duration of single antiplatelet therapy vs DAPT for 1 year) in these patients. The consistency of the treatment effect will be examined among 10 predefined relevant clinical subgroups (table 2).
Predefined relevant clinical subgroups
| Age |
| Sex |
| Diabetes |
| ST-segment elevation myocardial infarction vs other clinical presentations |
| SCAD angiographic pattern (angiographic dissection vs intramural hematoma) |
| Initial coronary revascularization |
| SCAD location (involved artery) |
| Multivessel SCAD |
| Left ventricular ejection fraction |
| Extracoronary vascular abnormalities, including fibromuscular dysplasia and others |
SCAD, spontaneous coronary artery dissection.
These predefined subgroups will be analyzed to determine formal interactions with the primary endpoint.
A number of secondary objectives will be also addressed using predefined ancillary studies.18–25 These prospective systematic substudies include: a) angiographic and quantitative coronary angiography analyses; b) coronary revascularization (selection of ideal candidates and optimization of the results of different coronary interventions; c) intracoronary imaging (optical coherence tomography and intravascular ultrasound); d) cardiac computed tomography and magnetic resonance imaging; e) assessment of extracoronary vascular abnormalities including fibromuscular dysplasia; f) inflammatory, immunologic, and micro-RNA analyses, and g) genetics and pharmacogenetics.
Sample size estimationRandomization will be performed as soon as possible after initial clinical stabilization (within 48hours of diagnosis) so that in-hospital events occurring after randomization, but before discharge, will be captured in the primary endpoint. The literature on in-hospital events is heterogeneous, including 1% mortality, 2% recurrent MI, and 5% to 10% of unplanned revascularization (nonexclusive events).1,2,5–8,13–16 It is estimated that half of the in-hospital events (∼5%) will be counted in the primary endpoint of the study.1,2,5–8 The available data on event rates after discharge and within 1 to 2 years is also highly heterogeneous partly because of inconsistent definitions and the inclusion of different individual endpoints. A study performed at the Mayo Clinic suggested an adverse event rate of 28% at 21 months.6 Overall, the expected event rate during this time period may be summarized as follows: recurrent SCAD 15%, recurrent MI 15%, stroke 1%, revascularization 10%, readmission for ACS 15%, readmission for heart failure 5%.1,2,5–8,13–16 However, we should acknowledge that readmission rates for ACS or heart failure remain unreported in most previous studies. Therefore, a total rate of 10% to 25% (estimated mean 12.5%) of major adverse cardiovascular events are expected at 1 year.
- •
Assuming an event rate (combined primary endpoint at 1 year) of 8% in the treatment arm (beta-blocker stratum) and of 15% in the no-treatment arm (relative risk reduction of 47%), 278 patients per arm (278 x 2=556 patients in total) will provide a power of 80% with 95% confidence intervals.
- •
This sample size will also allow adequate comparison of the arms of the antiplatelet stratum (assuming a 12% event rate for the primary endpoint in the prolonged DAPT arm with a 3% noninferiority margin in the 1-month single antiplatelet therapy).
According to these assumptions, 600 patients will be required (figure 2 and figure 3). Nevertheless, more accurate final decisions in this regard will be made following an adaptive flexible design according to real event results. The Steering Committee will decide on potential changes in the sample size if there is a lower than anticipated event rate. In this regard, the information accrued in this interim analysis will be used to inform Bayesian statistics and mathematical models and, eventually, to adjust the sample size as required. An independent and blinded Clinical Events Committee will adjudicate all adverse events after reviewing the corresponding anonymized source documents and images.
Routine care after ACS includes early electrocadiographic and hemodynamic monitoring and serial electrocardiograms and biomarkers to assess the extent of myocardial injury. Left ventricular function will be assessed before discharge. Hospitalization for 3 to 5 days will be recommended as a reasonable strategy to allow observation for adverse ischemic events.1,2 Clinical follow-up evaluations will be scheduled at 1 month, 3 months, 6 months, 1 year, and yearly thereafter.
Systematic screening for extracoronary vascular abnormalities including fibromuscular dysplasia is currently recommended in these patients.1,2 Individual sites should select the preferred screening strategy according to local resources, logistics, and experience. Notwithstanding the importance of these studies, they will not be mandated, given the pragmatic study design.
The study will allow the possibility of enhancing the information obtained on this disease by additional predefined multidisciplinary “ancillary” substudies. Accordingly, as part of a comprehensive and systematic approach to SCAD, the different centers will be invited to participate in several ancillary studies (clinical, revascularization, invasive and noninvasive imaging, biomarkers, novel inflammatory molecules and mRNAs, genetics, quality of life questionnaire), which will be carefully organized to provide a comprehensive and multidisciplinary view of this disease.18–25 However, these studies are not required by the main protocol and will only be performed as selected by the local investigators.
Statistical analysisFactorial designs allow efficient evaluation of multiple treatments within a single trial without increasing the sample size and ensuring that the treatments under evaluation work independently.26 In our study, there is an absence of biological plausibility for an interaction between the 2 strata (beta-blockers and antiplatelet agents). However, potential treatment interaction affecting the results of the trial will be evaluated using significance tests and by presenting the size of interaction term as a measure of uncertainty (95% confidence intervals). The study will also present results from a “multi-arm” analysis to allow further comparison between the 2 strata (and margins) independently. The study will be reported following the “modified” CONSORT guidelines for pragmatic trials, from the CONSORT and PRACTIHC (Pragmatic Trials in Healthcare Systems) groups, to better assess the applicability of the results.27 The extended 8 CONSORT checklist items for the reporting of pragmatic trials (background, participants, interventions, outcomes, sample size, blinding, participant flow, and generalizability of the findings) will be followed.27
As no interaction between the treatment strategies is expected, the factorial designed should not be statistically penalized regarding the statistical power.26 The main analysis is based on the time from randomization to the first occurrence of any component of the primary composite endpoint. The main analysis will be performed according to the intention-to-treat principle and will include all adjudicated endpoint events occurring from randomization to the end of the trial. Exploratory sensitivity analyses of the primary endpoint and the co-primary endpoint in the on-treatment and per protocol data set will be also performed.
Predefined hierarchical testing of ranked secondary endpoints will be performed. Cox-proportional hazard models and regression analyses will be used to identify independent predictors of adverse clinical outcomes. Hazard ratios (HR, 95% confidence intervals) will be calculated. Event-free survival will be analyzed using Kaplan-Meier curves and will be compared by the log-rank test. The number needed to treat will be established for the treatment modalities. A 2-tailed P value <.05 will be considered statistically significant.
DISCUSSIONThe aim of this study is to generate enough scientific evidence to inform clinical decisions and to address some of the unmet needs regarding medical therapy identified by the 2 recent international consensus documents on SCAD.1,2 As a corollary, the additional general clinical information obtained in this uniquely large cohort of patients with SCAD and in the predefined prospective substudies will shed new light on the pathophysiology, diagnosis, and treatment of this unique clinical condition.18–25
Research priorities according to consensus documentsThe unmet clinical need and research priorities in SCAD have been identified by recent expert consensus documents from both sides of the Atlantic.1,2 The recently reported Scientific Statement on SCAD of the American Heart Association highlights the importance of addressing major persistent knowledge gaps on this clinical entity.1 The document states that, as most currently available information comes from retrospective observational studies, there is high risk of gender bias, referral bias, and survivor bias. This suggests that the incidence and recurrence risk of SCAD are likely underestimated. Therefore, major efforts should be made to provide robust evidence-based knowledge on this “orphan” disease. The American Heart Association statement calls for large-scale prospective studies to gain insight on treatment options and prevention of recurrences. Regarding medical therapy, due to the lack of controlled trials to support an evidence-based approach, recommendations “are based largely on expert opinions derived from clinical experience of members of the writing group”. The writing committee specifically identified that a major remaining question is optimal medical therapy, specifically, the value of beta-blockers and antiplatelet therapy. Currently, there are no data from prospective controlled studies supporting the value of beta-blockers or antiplatelet agents in SCAD patients. A strong “call for action” was issued to initiate collaborative research efforts in this regard.1
The European Society of Cardiology recently issued a position paper on SCAD.2 The first sentence on medical management in this document clearly underscores the unmet needs in this challenging condition: “There are to date no randomized clinical trials comparing different pharmacological treatment strategies for SCAD”. Regarding antiplatelet therapy, this document further states “The use of antiplatelet therapies and the duration of treatment remains an area of controversy and divergent practice”. With respect to the use of beta-blockers, the document states the following: “more controversial is the management of SCAD-survivors in patients without significant impairment of left ventricular systolic function”. This European Society of Cardiology position paper identifies major research priorities in SCAD, highlighting that “there is no specific disease-modifying therapy”. In addition, it also declares: “prospective studies (including ultimately randomized studies) assessing the best medical therapies (eg, role and duration of antiplatelet therapy, use of beta-blockers) … are urgently needed”.2
Assessing rare diseases and gender biasSCAD is typified as “rare disease” according to EURORDIS (ORPHA:458718). Promoting the study of rare diseases, as a public health priority, is of paramount importance considering that—by definition—medical expertise is rare, knowledge scarce, care offerings inadequate, and research very limited. Despite their large overall number, patients with rare diseases are the orphans of health systems, often denied diagnosis, treatment, and the benefits of research. Nationwide prospective initiatives, such as the current project, will help to advance our knowledge on SCAD. The controlled design of the BA-SCAD trial will shed important light on this unique condition and will provide evidence-based knowledge to inform everyday clinical decisions.
In addition, major scientific and academic groups highlight the importance of research initiatives in women, considering the existence of a major gender bias in most areas of medicine. Women are systematically underrepresented in clinical trials. This is more than evident in studies on coronary artery disease, ACS, and MI. Notably, recent information suggests that up to 25% of MI in premenopausal women is caused by SCAD.1,2 Unfortunately, the diagnosis remains frequently overlooked. The current study will further expand our understanding of the pathophysiology of SCAD (a disease disproportionately affecting female patients) and ACS in women.
Rationale and feasibilityThe rationale of this trial is clear. In brief, beta-blockers have been approved by clinical practice guidelines for the treatment of patients with MI.3,4 These drugs reduce total and cardiovascular mortality after MI.3,4,28,29 Beta-blockers are also frequently used in SCAD patients to reduce coronary shear stress, blood pressure, and myocardial contractility and aim to mitigate coronary injury and further vessel wall disruption.1,2 However, these drugs may predispose patients to coronary spasm and might even be detrimental in patients with SCAD as the result of their effects on the injured vessel wall and the reduced residual coronary lumen. Beta-blockers with alpha-blocker activity are particularly attractive to avoid enhancing the risk of coronary spasm and, therefore, should be considered in these patients. Alternatively, the use of DAPT for 1 year is the guideline-recommended therapy for patients with ACS as a result of atherosclerotic coronary artery disease.3,4 Accordingly, antiplatelet drugs are also frequently prescribed, albeit with a variable duration, in patients with SCAD.1,2 However, in SCAD, intramural bleeding appears to be the initiating pathophysiological event and, therefore, this therapy might be of no value or even deleterious. Whether SCAD patients benefit from the general recommendations for ACS patients (namely prolonged DAPT) or, due to a unique underlying pathophysiology, they are better off with a very limited antiplatelet strategy is currently unknown. Nowadays, both the prescription and nonprescription of beta-blockers and the use of single antiplatelet therapy or DAPT (with variable duration), are valid options in the management of patients with SCAD.1,2 This is an area of intense controversy not only in the scientific community but also in routine clinical practice.1,2
Many practical issues might affect the external validity of the trial results to the general population. The requirement to clinically stabilize the patients before randomization may induce a selection bias by excluding sicker patients with SCAD. Accordingly, a registry will be kept of SCAD patients not randomized in this trial. Some members of the research team are leading experts in the field of beta-blocker therapy in the acute and chronic MI setting and have gained experience in actions to reduce the risk associated with lack of compliance and crossovers in pragmatic open-label trials using beta-blockers. Based on previous experience, we are confident that the type and dose of beta-blocker prescribed in patients randomized to active treatment will not significantly impact the study results.29 During follow-up, we will keep track of the agent and dose being taken by the patient at each follow-up visit. An Achilles heel of open-label randomized clinical trial is the possibility of having a high rate of crossovers. This is especially true when the medications being tested are highly prescribed. Major care will be used to ensure treatment adherence and address the implications of crossovers. Patients will be contacted by telephone immediately after discharge and at 1 month to remind them that if any physician asks for a change in the allocated treatment, it is desirable that they contact the research team for discussion. The low-intervention and pragmatic trial design coupled with a carefully organized clinical follow-up (for adherence and compliance) will be of major value to ensure follow-up completeness.
According to the inclusion and exclusion criteria and data from the Spanish population using the International Classification of Diseases, Ninth Revision (ICD-9) the incidence SCAD in Spain was estimated. Data from the Spanish Ministry of Health (available from the Spanish Society of Cardiology and IMAS Foundation) suggests a stepwise increase in the number of patients diagnosed with SCAD each year in Spain (187 patients in 2005, 288 patients in 2010, 650 patients in 2015). The reasons explaining this increment may include increased awareness of the disease and unrestricted use of early coronary angiography for ACS patients. Considering this trend, it is estimated that ∼900 SCAD cases will be diagnosed yearly during the study period (2021-2023). Accordingly, and considering the pragmatic, all-comers study design, it is assumed that 300 patients will be enrolled trial in the first year (2021-2022) and 300 patients in the second (2022-2023) in the present trial (figure 1). Data from the Spanish SCAD registry5,30 (currently 30 active sites) suggests that 60 centers (each enrolling a mean of 5 SCAD patients/year) will be required to achieve the estimated sample size. All centers participating in the SCAD registry have agreed to participate in the trial (). However, the number of centers will be increased and modified as required according to the actual enrollment data.
CONCLUSIONSThe role of beta-blockers and antiplatelet agents in SCAD remains to be established. Given the extreme clinical relevance of this burning therapeutic question, a prospective randomized clinical study is needed. We are certain that this uniquely designed pragmatic randomized clinical trial (BA-SCAD) will advance our knowledge and inform the medical management of this challenging clinical entity. The suggested integrated approach, involving multidisciplinary ancillary substudies, will also provide important insights on the pathophysiology of SCAD and lay the foundations for future research in the field.
FUNDINGThis study will be possible due to the support of the Spanish Society of Cardiology (ECAM [Ensayo Clinico Aleatorizado Multicéntrico] Award 2021).
AUTHORS’ CONTRIBUTIONSAll authors have participated in the design of the study, in the writing and supervision of this article and have approved the final version of the manuscript.
CONFLICTS OF INTERESTD.J. Angiolillo declares that he has received consulting fees or honoraria from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company and has received payments for participation in review activities from CeloNova and St Jude Medical, outside the present work. D.J. Angiolillo also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co, Merck, Novartis, Osprey Medical, Renal Guard Solutions and Scott R MacKenzie Foundation. The remaining authors declare that they have no conflicts of interest in relation to this work.
- -
SCAD is a rare but increasingly recognized cause of ACS predominantly affecting young to middle-aged women. The pathophysiology of SCAD is unique and differs from that seen in patients with ACS resulting from complicated atherosclerotic coronary plaque. Most patients with SCAD are initially managed conservatively and treated with beta-blockers and antiplatelet drugs. However, the evidence supporting the clinical value of these drugs in patients with SCAD is very limited.
- -
The BA-SCAD clinical trial, is an academic, pragmatic, multicenter, randomized factorial (1:1/1:1) study, performed under the auspices of the Spanish Society of Cardiology (promoter), to assess the efficacy of pharmacological therapy (namely beta-blockers and antiplatelet agents) in patients with SCAD. The primary efficacy endpoint includes a composite of death, myocardial infarction, stroke, coronary revascularization, recurrent SCAD, and unplanned hospitalization for ACS or heart failure at 1 year. The trial will generate the required scientific evidence to inform clinical decisions to select the optimal medical regimen in these challenging patients.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.08.003