
The role of [18F]FDG-PET/CT in cardiac implantable electronic device (CIED) infections requires better evaluation, especially in the diagnosis of systemic infections. We aimed to determine the following: a) the diagnostic accuracy of [18F]FDG-PET/CT in each CIED topographical region, b) the added value of [18F]FDG-PET/CT over transesophageal echocardiography (TEE) in diagnosing systemic infections, c) spleen and bone marrow uptake in differentiating isolated local infections from systemic infections, and d) the potential application of [18F]FDG-PET/CT in follow-up.
MethodsRetrospective single-center study including 54 cases and 54 controls from 2014 to 2021. The Primary endpoint was the diagnostic yield of [18F]FDG-PET/CT in each topographical CIED region. Secondary analyses described the performance of [18F]FDG-PET/CT compared with that of TEE in systemic infections, bone marrow and spleen uptake in systemic and isolated local infections, and the potential application of [18F]FDG-PET/CT in guiding cessation of chronic antibiotic suppression when completed device removal is not performed.
ResultsWe analyzed 13 (24%) isolated local infections and 41 (76%) systemic infections. Overall, the specificity of [18F]FDG-PET/CT was 100% and sensitivity 85% (79% pocket, 57% subcutaneous lead, 22% endovascular lead, 10% intracardiac lead). When combined with TEE, [18F]FDG-PET/CT increased definite diagnosis o fsystemic infections from 34% to 56% (P=.04). Systemic infections with bacteremia showed higher spleen (P=.05) and bone marrow metabolism (P=.04) than local infections. Thirteen patients without complete device removal underwent a follow-up [18F]FDG-PET/CT, with no relapses after discontinuation of chronic antibiotic suppression in 6 cases with negative follow-up [18F]FDG-PET/CT.
ConclusionsThe sensitivity of [18F]FDG-PET/CT for evaluating CIED infections was high in local infections but much lower in systemic infections. However, accuracy increased when [18F]FDG-PET/CT was combined with TEE in endovascular lead bacteremic infection. Spleen and bone marrow hypermetabolism could differentiate bacteremic systemic infection from local infection. Although further prospective studies are needed, follow-up [18F]FDG-PET/CT could play a potential role in the management of chronic antibiotic suppression therapy when complete device removal is unachievable.
Keywords
Cardiac implantable electronic devices (CIED) figure in a broad clinical spectrum of infections, such as local CIED infections, which can appear as isolated local infections (LI) or associated with systemic lead infections (SI). SI involve endovascular lead and intracardiac lead infections, including infective endocarditis (IE). General diagnosis is challenging and is based on microbiological data and cardiac imaging techniques such as transesophageal echocardiography (TEE).1–3,518F-fluorodeoxyglucose-positron emission tomography/computed tomography ([18F]FDG-PET/CT) has improved the diagnostic evaluation of prosthetic valve endocarditis and has been incorporated as a major diagnostic criterion in guidelines.1 In addition, it has been recently shown that hypermetabolism of the spleen and bone marrow (BM) as detected by [18F]FDG-PET/CT can be considered an indirect sign of IE in native or prosthetic valves.4,6
Despite the latest evidence, the overall usefulness of [18F]FDG-PET/CT in CIED infections requires further characterization. Several cohort studies have been published,3,5 showing high diagnostic yield for generator pocket infections but much lower performance in lead-associated infection (SI).7 TEE is also unable to detect lead vegetations in many patients with bacteremia, who probably have an endovascular lead infection (SI).2 [18F]FDG-PET/CT could help to improve the diagnosis in all topographical regions of CIEDs, including in endovascular leads, which cannot be accessed by TEE.
The primary endpoint of this study was to determine the diagnostic yield of [18F]FDG-PET/CT in each of the different CIED topographical regions: pocket, subcutaneous, endovascular, and intracardiac leads. Secondary endpoints were to analyze the performance of [18F]FDG-PET/CT compared with that of TEE in diagnosing SI, to define the diagnostic value of spleen and BM hypermetabolism as an indirect sign of SI, and to assess the potential usefulness of [18F]FDG-PET/CT in the follow-up of CIED infections without complete device removal and suppressed with chronic antibiotics to avoid relapses, guiding physicians on when to stop chronic oral suppression (CAS) therapy.
METHODSStudy designA retrospective case-control study was conducted at Hospital Clínic de Barcelona, a referral center for IE and cardiovascular infections, assessed by the members of the Hospital Clinic of Barcelona Infective endocarditis Team investigators (see for a list of the investigators) to evaluate the usefulness of [18F]FDG-PET/CT in the diagnosis of CIED infections. All suspected cases of CIED infection have been discussed during weekly IE team meetings since 1986.8 The final diagnosis of each case was reached through the application of the modified Duke criteria9 and international guidelines2 by consensus. We included all consecutive patients with definite CIED infection who met the inclusion criteria from January 2014 to January 2021. Information was gathered from the electronic medical clinical data. Consecutive cases were matched with controls by age (± 5 years), sex, CIED type, and calendar year. All patients were followed up for at least 1 year until December 2021.
Inclusion criteriaCases (true positives)Local and systemic infections were classified following European Heart Rhythm Association (EHRA) diagnosis criteria recommendations.2 For suspected cases of CIED-IE, the modified Duke criteria were applied.9 In all cases, LI and SI were evaluated by performing blood cultures, swab, pocket (device and leads when extracted) cultures and 16SrRNA-PCR, and echocardiography. For the primary objective of this study, ie, evaluating the diagnostic accuracy of [18F]FDG-PET/CT (sensitivity, specificity, positive and negative predictive value), [18F]FDG-PET/CT results were excluded as a major diagnostic criterion in cases. All CIED infections were surveyed using this imaging modality.
The final diagnosis was achieved by consensus of the weekly IE team meetings for each case. Only patients with a definite diagnosis of CIED infection were included.
Types of CIED infectionIsolated local device infectionsLocal signs of infection were those involving the pocket generator with or without subcutaneous lead, and/or positive cultures of pocket swab, device, subcutaneous lead (and positive 16SrRNA-PCR when performed). This group included definitions of CIED-related infection as specified in the EHRA consensus: isolated generator pocket infection, isolated pocket erosion, pocket site infection without bacteremia/systemic signs of infection.2
We defined isolated LI as those not associated with systemic signs of infection. Patients with suspicion of SI or positive endovascular/intracardiac lead culture were systematically excluded from this group.
Systemic infectionsSI were those occurring in patients with or without associated local CIED infection who also had endovascular/intracardiac lead infection (including IE) determined by systemic signs of infection, eg, fever, elevated C-reactive protein, leukocytosis, and positive blood cultures or endovascular/intracardiac lead cultures (and positive 16SrRNA-PCR when performed), and/or the presence of vegetations on leads or the tricuspid valve, diagnosed by TEE. This group included definitions of CIED-related infection as clarified in the EHRA consensus: lead infection, pocket site infection with lead/valvular endocarditis, CIED endocarditis without pocket infection, positive blood cultures, and lead or valvular vegetations.2 Patients classified as having possible or probable SI were excluded, because they were not considered as definite true positives.
Controls: true negativesPatients with CIED and studied by [18F]FDG-PET/CT due to solid or hematologic neoplasms were included as controls without indication of CIED FDG uptake status. All the topographical regions of the control CIEDs were evaluated, except the intracardiac lead segment, as none of the controls underwent myocardial uptake suppression.10
Matching criteriaAll cases and controls were paired by age, sex, type of device, and similar time interval between CIED implant/replacement and [18F]FDG-PET/CT performance.
Exclusion criteriaCasesWe excluded patients with no definite criteria of CIED infection. As mentioned above, all cases were considered as true positive; there were no false positives.
ControlsWe excluded patients with previous CIED infections or any clinical or laboratory sign of local or systemic infection within the 6 months before or after the moment of [18F]FDG-PET/CT acquisition. We also excluded patients with central intravenous lines and/or mediastinal hypermetabolic lesions that could interfere with the assessment.
[18F]FDG-PET/CT considerationsWhole-body [18F]FDG-PET/CT scans were acquired 60minutes after [18]F-FDG injection (4.0 MBq/kg) in a hybrid scanner (Biograph mCT 64S; Siemens, Germany) with a myocardial uptake suppression protocol consisting of a 12-hour fasting period and intravenous administration of 50 IU/kg of unfractionated heparin 15minutes before [18]F-FDG injection. Diabetic patients were managed as indicated by EANM/SNMMI guidelines for [18]F-FDG use in inflammation and infection.6,10 Consuming a high-fat, low-carbohydrate diet before [18F]FDG-PET/CT scanning was not systematically introduced in all patients, given that this protocol was implemented after we designed the study.
Visual analysisAll patients underwent whole-body [18F]FDG-PET/CT as a part of the study protocol. The primary endpoint was the [18F]FDG-PET/CT result, which was assessed qualitatively by 2 blinded, independent nuclear medicine specialists. All images were interpreted separately by the 2 independent nuclear medicine specialists, and disagreements were settled by consensus with a third nuclear medicine reader. The positivity criterion was the presence of any focal or heterogeneous uptake related to each topographical region identified in both attenuation-corrected and uncorrected images to avoid attenuation-correction artifacts. The results of [18F]FDG-PET/CT visual analysis were also compared with those of TEE in SI.
Semiquantitative analysisSemiquantitative analysis, supervised by both readers, was performed in all [18F]FDG-PET/CT scans by measuring the maximum standardized uptake value (SUVmax) of a volume of interest sphere including the totality of the pocket and a volume of interest sphere placed on the most active part of each segment of the lead (subcutaneous, endovascular, and intracardiac).
No semiquantitative analysis was performed in the intracardiac lead regions of control participants as they did not undergo the myocardial inhibition protocol. Hence, specificity analysis for intracardiac lead was excluded from the statistical analysis.
Spleen and bone marrow metabolismValues of SUVmean were obtained for spleen and BM to assess indirect signs of infection/inflammation as described by Boursier et al.6 by placing a spherical volume of interest at the center of the spleen and in 1 lumbar vertebra, carefully avoiding the inclusion of any abnormal area secondary to possible lesions. For reference, descending thoracic aorta blood pool-SUVmean was calculated as was liver SUVmean. SUV ratios were calculated by dividing the SUVmax of the area of interest by the blood pool and liver-SUVmean with the aim of overcoming any bias related to individual physiological fluctuations of [18]F-FDG distribution.
Follow-up [18F]FDG-PET/CTAt least 1 [18F]FDG-PET/CT scan within the first 6 months after discharge was achieved in all patients with incomplete device removal. At least 1 [18F]FDG-PET/CT scan was scheduled every 4 to 6 months; more than 1 [18F]FDG-PET/CT scan may have been performed depending on the length of follow-up completed during the study. Data on chronic antibiotic suppression (CAS) therapy and its duration, as well as type of infection, were also analyzed. Further details regarding [18F]FDG-PET/CT methodology can be found in the .
Transesophageal echocardiographyEchocardiographic assessment was achieved by TEE in all cases using a GE VIVID E95 system. Any mass seen on a lead in echocardiography in the context of bacteremia was assumed to be vegetation. All echocardiography exams were validated by a second investigator, and further discrepancies by a third member of the team.
Statistical analysisContinuous variables are presented as median [interquartile range] and were compared using the Mann-Whitney U-test. Categorical variables are presented as frequencies (percentages) and were compared using the chi-square or Fisher test. For all tests, statistical significance was set at P<.05. Validity calculations of sensitivity, specificity, and positive and negative predictive values were obtained using contingency tables according to the true positive and true negative, false positive and false negative results obtained from [18F]FDG-PET/CT results. Receiver operating characteristics (ROC) curves were also performed from the different SUVmax/mean values to obtain a more accurate cutoff point for diagnosis of infection. Statistical analyses were conducted with STATA 14.0.
Ethical considerationsThe implementation of this study was approved by the Ethics Review Board of Hospital Clínic de Barcelona (Ethics Review Board number HCB/2020/1489). The requirement for written informed consent was waived given the retrospective nature of the study. Patient identification was encoded, complying with the requirements of the Organic Law on Data Protection 15/1999.
RESULTSWe included 54 cases and 54 controls; the characteristics of the 2 groups are presented in table 1. In 25% of cases, less than 152 days elapsed between the implant or device change procedure and the clinical infection.
Baseline characteristics of cases (CIED infections) and controls
| Casesn=54 | Controlsn=54 | P | |
|---|---|---|---|
| Variables | |||
| Age, y | 78[69.0-85.0] | 83[77.0-88.0] | - |
| Female sex | 16(29.6) | 10(18.5) | |
| Days between CIED implantation/replacement and [18F] FDG PET/CT | 768.5 [152.0-2443.0] | 1389.0 [707.0-3131.0] | <.01 |
| CIED type | |||
| Pacemaker | 41 (75.9) | 44 (81.5) | - |
| ICD | 12 (22.2) | 10 (18.5) | - |
| CRT | 1 (1.9) | 0 | - |
| [18F] FDG PET/CT results | |||
| Positive [18F] FDG PET/CT | 46 (85.2) | 0 | - |
[18F]FDG-PET/CT, 18F-fluorodeoxyglucose-positron emission tomography/computed tomography; CIED, cardiac implantable electronic device; CRT, cardiac resynchronization therapy; ICD, implantable cardiac defibrillator.
The data are expressed as No. (%) or median [interquartile range].
Comparison between cases with isolated local infection and those with systemic infection or both types of infection
Cases were divided into those with isolated LI (n=13) and those with SI with or without local infection (n=41). Baseline characteristics were similar between the w2 groups (table 2). Local signs of device infection were present in 87% (47/54) of cases: 100% (13/13) with isolated LI and in 82.9% (34/41) of those in the SI group (P <.01). Of the patients with SI, 34.1% (14/41) had a positive echocardiography result. Microbiological positivity and etiology were distributed homogeneously in the 2 groups, with a predominance of Staphylococcus aureus and coagulase-negative staphylococci (CNS) (). The specific classification of SI in terms of the diagnostic criteria is summarized in . Patients with SI underwent significantly more removal surgery (70.7% vs 38.4%; P=.04); those with isolated LI received more CAS (61.5% vs 24.4%; P <.01). There were no statistically significant differences between patients with isolated LI and SI regarding reimplant surgery, in-hospital mortality, or relapse. There were no differences in [18F]FDG-PET/CT results globally or for any topographical segment during the interval between CIED implant/replacement and [18F]FDG-PET/CT (< 3 months vs> 3 months). All characteristics comparing groups and [18F]FDG-PET/CT results are summarized in .
Comparison of patients with CIED infection by isolated local or systemic infections
| Total | Isolated local infectionsn=13 | Systemic infectionsn=41 | P | |
|---|---|---|---|---|
| Baseline and matching characteristics | ||||
| Age, y | 78[69.0-85.0] | 83.0[75.0-87.0] | 77.0[69.0-85.0] | .35 |
| Female sex | 16(29.6) | 4(30.7) | 12(29.2) | .91 |
| CIED type | ||||
| Pacemaker | 41(75.9) | 9(69.2) | 32(78) | .54 |
| ICD | 12(22.2) | 4(30.7) | 8(19.5) | .42 |
| CRT | 1(1.9) | 0 | 1(2.4) | .31 |
| Local infection signs | 47(87) | 13(100) | 34(82.9) | <.01 |
| Echocardiography | ||||
| Echo vegetation (TTE/TEE)* | 14(25.9) | 0 | 14(34.1) | NA |
| Lead vegetation | 14(25.9) | 0 | 14(34.1) | NA |
| Tricuspid valve vegetation | 2(3.7) | 0 | 2(4.8) | NA |
| Mitral valve vegetation | 1(1.8) | 0 | 1(2.4) | NA |
| [18]FDG-PET/CT | ||||
| Positive [18F]FDG-PET/CT | 46(85.2) | 11(84.6) | 35(85.3) | .94 |
| 37 | 8 | 29 | .54 | |
| Subcutaneous lead | 27 | 7 | 20 | .75 |
| Endovascular Lead | 9 | 0 | 9 | NA |
| Intracardiac lead | 4 | 0 | 4 | NA |
| Systemic emboli | 1(1.8) | 0 | 1(2.4) | NA |
| Pulmonary emboli | 1(1.8) | 0 | 1(2.4) | NA |
| Interval between CAS initiation and [18F]FDG-PET/CT, d | 6.0[0.0-14.0] | 6.0[0-15.0] | 8.0[4.0-13.0] | .38 |
| Interval between in-hospital admission and device removal, d | 8.5[1.5-14.0] | 5.5[1.0-14.0] | 12.5[6.0-14.0] | .25 |
[18F]FDG-PET/CT, 18F-fluorodeoxyglucose-positron emission tomography/computed tomography; CAS, chronic antibiotic suppression, CIED, cardiac implantable electronic device; CRT, cardiac resynchronization therapy; ICD, implantable cardiac defibrillator; NA, not available.
The data are expressed as No. (%) or median [interquartile range].
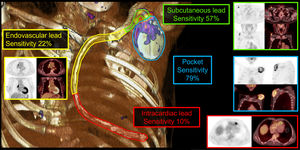
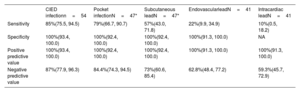
The main results can be found in table 3. The overall sensitivity of [18F]FDG-PET/CT for confirmed CIED infection was 85% (46/54). Pocket sensitivity was 79% (37/47), subcutaneous lead 57% (27/47), endovascular lead 22% (9/41), and 10% (4/41) intracardiac lead. However, intracardiac lead sensitivity might be underestimated because 31.5% (17/54) of cases showed unsuccessful myocardial inhibition. The negative predictive value was 15% (8/54). Median time on antibiotic treatment before [18F]FDG-PET/CT acquisition was 5 [0-14] days in cases with positive results and 13 [5–16] days in cases with negative results (P=.19). Despite the existence of a trend, there were no significant differences were found regarding the period between antibiotic was initiated and [18F]FDG-PET/CT performance; 12 (22.2%) cases had been on antibiotic therapy prior to [18F]FDG-PET/CT acquisition with a median duration of 6 [0.0–14.0] days.
Overall diagnostic accuracy of [18F]FDG-PET/CT according to the 4 topographical regions of CIED infection
| CIED infectionn=54 | Pocket infectionN=47* | Subcutaneous leadN=47* | EndovascularleadN=41 | Intracardiac leadN=41 | |
|---|---|---|---|---|---|
| Sensitivity | 85%(75.5, 94.5) | 79%(66.7, 90.7) | 57%(43.0, 71.8) | 22%(9.9, 34.9) | 10%(0.5, 18.2) |
| Specificity | 100%(93.4, 100.0) | 100%(92.4, 100.0) | 100%(92.4, 100.0) | 100%(91.3, 100.0) | NA |
| Positive predictive value | 100%(93.4, 100.0) | 100%(92.4, 100.0) | 100%(92.4, 100.0) | 100%(91.3, 100.0) | 100%(91.3, 100.0) |
| Negative predictive value | 87%(77.9, 96.3) | 84.4%(74.3, 94.5) | 73%(60.6, 85.4) | 62.8%(48.4, 77.2) | 59.3%(45.7, 72.9) |
CIED, cardiac implantable electronic device; NA, not available; LI, isolated local infection.
Figure 1 shows positive [18F]FDG uptake examples and sensitivity values of FDG-PET/CT in a visual 3-dimensional representation of each CIED topographical region.
Central illustration. The figure shows examples of positive FDG uptake and sensitivity values of [18F]FDG-PET/CT in a 3D visual representation of each CIED topographical region: pocket (blue), subcutaneous (green), endovascular (yellow), and intravascular (red). 3D, three-dimensional; [18F]FDG-PET/CT, 18F-fluorodeoxyglucose-positron emission tomography/computed tomography. CIED, cardiac implantable electronic-device.
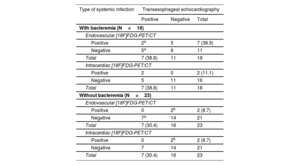
Table 4 compares diagnostic performance between TEE and [18F]FDG-PET/CT in patients with systemic infection showing fever, leukocytosis and elevated C-reactive protein with positive blood cultures or positive lead cultures/16SrRNA-PCR and/or positive echo. In those patients, when [18F]FDG-PET/CT was combined with TEE, the definite diagnosis rate of infection significantly increased from 34% (14/41) to 56% (23/41) (P=.04) due to detection of endovascular involvement, with rates higher in the bacteremic (from 38.8% ([7/18] to 66.7% [12/18]) than in the nonbacteremic form (from 30.4% ([7/23] to 47.8% [11/23]) of systemic infections (P=.37).
Diagnostic performance of [18F]FDG-PET/CT
| Type of systemic infection | Transesophageal echocardiography | ||
|---|---|---|---|
| Positive | Negative | Total | |
| With bacteremia (N=18) | |||
| Endovascular [18F]FDG-PET/CT | |||
| Positive | 2a | 5 | 7 (38.9) |
| Negative | 5a | 6 | 11 |
| Total | 7 (38.8) | 11 | 18 |
| Intracardiac [18F]FDG-PET/CT | |||
| Positive | 2 | 0 | 2 (11.1) |
| Negative | 5 | 11 | 16 |
| Total | 7 (38.8) | 11 | 18 |
| Without bacteremia (N=23) | |||
| Endovascular [18F]FDG-PET/CT | |||
| Positive | 0 | 2b | 2 (8.7) |
| Negative | 7a | 14 | 21 |
| Total | 7 (30.4) | 16 | 23 |
| Intracardiac [18F]FDG-PET/CT | |||
| Positive | 0 | 2b | 2 (8.7) |
| Negative | 7 | 14 | 21 |
| Total | 7 (30.4) | 16 | 23 |
[18F]FDG-PET/CT, 18F-fluorodeoxyglucose-positron emission tomography/computed tomography.
Values are expressed as absolute numbers or No. (%).
Diagnostic performance of [18F]FDG-PET/CT compared to transesophageal echocardiography in 41 patients with systemic infection with (18 patients) or without (23 patients) bacteremia.
ROC curves were analyzed for the median SUVmax of all 4 CIED topographical regions and the ratio between each SUVmax/liver SUVmean and blood pool-SUVmean. Clinically significant values were only found in pocket uptake for SUVmax and SUVmax/SUVmean liver values, it is shown in figure 2. The remaining ROC curves can be found in .
A: ROC curve for CIED pocket SUVmax cutoff point 2.35 [sensitivity: 79.63%; 92.59%]. B: ROC curve for CIED pocket SUVmax/SUVmean liver, cutoff point 1.28 [sensitivity: 75.56%; specificity: 88.89%]. CIED, cardiac-implantable-electronic-device; ROC, SUV, standardized uptake value, SUVmax, maximum standardized uptake value.
There were no differences among any of the semiquantitative variables in cases and controls regarding spleen or BM uptake, including between LI and SI (). However, in the SI bacteremia subgroup, the SUVmean spleen (P=.05) and BM (P=.04) were significantly higher than in LI. These data are summarized in table 5.
Comparison of spleen and bone marrow SUVmean in cases of bacteremia
| SUVmean spleen | SUVmean bone marrow lumbar column | |
|---|---|---|
| Bacteremia SI vs LI vs controls | ||
| Bacteremia | 2.00 [1.7-2.3] | 1.75 [.6-1.9] |
| P value vs LI | .05 | .04 |
| P value vs controls | .43 | .71 |
SI, systemic infection; LI: isolated local infection.
Unless otherwise indicated, the values are expressed as median [interquartile range].
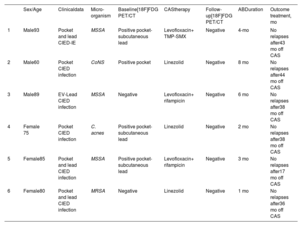
The overall cohort flowchart focused on patients with incomplete device removal who received CAS and underwent follow-up [18F]FDG-PET/CT is shown in . Complete system removal was performed in 66.7% (36/54) of cases and was significantly higher (P=.03) in patients with SI (73.1% [30/41]) than in those with isolated LI (46.2% [6/13]) (). Eighteen cases were classified as nonremoval or incomplete device removal (9/18 and 9/18, respectively). The main reasons for not removing devices were advanced age, severe comorbidities, patient frailty, and high surgical risk. Device removal was achieved in 45/54 (83.3%) of patients. Among patients who underwent device removal, the procedure was incomplete in 9/45 (20%). Most cases underwent manual traction (40/45 [88.9%]), whereas only 5/45 (11.1%) cases required open surgery. After hospital discharge, follow-up lasted for at least 6 months in all patients and a follow-up [18F]FDG-PET/CT was performed 13 patients (13/18) (65%). Two patients, who did not undergo follow-up [18F]FDG-PET/CT, died during hospital admission. The remaining 3 patients were followed up in other hospitals without [18F]FDG-PET/CT. Except the 2 patients who died before discharge, all patients (n=13) received CAS. The characteristics of the 18 patients without device removal can be found in . All patients underwent at least 1 [18F]FDG-PET/CT study; 4/13 patients underwent more than 3 [18F]FDG-PET/CTs during follow-up. The number of scans performed in each patient varied during follow-up, as they were indicated by the IE team on an individual basis for each case. Six patients switched from positive to negative FDG uptake during the follow-up, and 4 of them (66.7%) stopped CAS with IE Team agreement. Four patients with a previous negative [18F]FDG-PET/CT remained negative during the follow-up; 2 of them (50%) stopped CAS with IE Team decision. To date, there have been no signs of relapse in any of these 6 cases. The median time to a negative [18F]FDG-PET/CT result was 2 [1-5] months. The median follow-up time was 38 months; patients who interrupted CAS are shown in table 6.
Patients with incomplete device removal
| Sex/Age | Clinicaldata | Micro-organism | Baseline[18F]FDG PET/CT | CAStherapy | Follow-up[18F]FDG PET/CT | ABDuration | Outcome treatment, mo | |
|---|---|---|---|---|---|---|---|---|
| 1 | Male93 | Pocket and lead CIED-IE | MSSA | Positive pocket-subcutaneous lead | Levofloxacin+ TMP-SMX | Negative | 4-mo | No relapses after43 mo off CAS |
| 2 | Male60 | Pocket CIED infection | CoNS | Positive pocket | Linezolid | Negative | 8 mo | No relapses after44 mo off CAS |
| 3 | Male89 | EV-Lead CIED infection | MSSA | Negative | Levofloxacin+ rifampicin | Negative | 6 mo | No relapses after38 mo off CAS |
| 4 | Female 75 | Pocket CIED infection | C. acnes | Positive pocket-subcutaneous lead | Linezolid | Negative | 2 mo | No relapses after38 mo off CAS |
| 5 | Female85 | Pocket and lead CIED infection | MSSA | Positive pocket- subcutaneous lead | Levofloxacin+ rifampicin | Negative | 3 mo | No relapses after17 mo off CAS |
| 6 | Female80 | Pocket and lead CIED infection | MRSA | Negative | Linezolid | Negative | 1 mo | No relapses after36 mo off CAS |
C. acnes, Cutibacterium acnes; CAS, chronic antibiotic suppression, CIED-IE, cardiac implantable electronic device infective endocarditis; CoNS, coagulase negative staphylococci; EV, endovascular; MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus, subcutaneous lead; TMP-SMX, trimethoprim-sulfamethoxazole.
Patients with incomplete device removal on CAS therapy whose treatment was stopped according to the follow-up [18F]FDG-PET/CT result. The overall incomplete device removal in patients on CAS therapy is summarized in .
Several cohort studies of CIED infections have been published in recent years,7,11,12 reporting high sensitivity and specificity values for [18F]FDG-PET/CT in pocket infections but lower diagnostic performance in lead-associated infections. However, to date there is no gold standard for assessing the subcutaneous and endovascular lead portion in CIED infections. In addition, differentiation between LI and SI may be problematic, as intraoperative lead contamination in patients with LI might occur during device extraction.2–4,11.
In our study, [18F]FDG-PET/CT demonstrated an overall sensitivity for CIED infections of 85%: 79% for pocket infections, and 57% for subcutaneous lead infections. In contrast, in line with previous studies,7,12 our results showed low sensitivity on endovascular (22%) and intracardiac leads (10%). The specificity of [18F]FDG-PET/CT was 100% for all segments except intracardiac lead, which could not be evaluated, as there were no true negative intracardiac lead controls because none of the control patients underwent myocardial uptake suppression protocol.
Spread of the infection from a contaminated generator pocket through the subcutaneous lead into the endovascular space has been hypothesized to be the main pathogenic mechanism in CIED infections.4 This mechanism may explain 83% (34/41) of our SI cases. In addition, in our data, the [18F]FDG-PET/CT CIED pocket was the most frequent area of positive uptake, followed by subcutaneous lead. Nonetheless, Rizwan et al.10 suggests that CIED lead infection can also originate from a distant source, possibly explaining the remaining 7 cases (17%) with SI but without LI.
Compared with previous studies, our work shows equivalent sensitivity and specificity values with a larger sample of patients. In our cohort, the ROC curve for pocket SUVmax had a cutoff point of 2.4 with sensitivity of 79.6% and specificity of 92.6% (figure 2A). Other studies have reported similar results for diagnostic yield in pocket CIED infections.12–15 In contrast, Mahmood et al.7 showed higher sensitivity and specificity values for SI, probably due to a meta-analysis based on several heterogeneous studies with a small number of patients, divergent designs, and the inclusion of other prosthetic infections.
Eight out of 47 cases with LI showed normal [18F]FDG-PET/CT results considered as false negatives. In all but 1 false negative result, the patients had undergone antibiotic therapy for more than 20 days before [18F]FDG-PET/CT acquisition. Several studies have suggested that antibiotic therapy for more than 7 days before [18F]FDG-PET/CT acquisition can reduce its diagnostic performance.11,12,16 However, no significant differences were found in our cohort in the period between antibiotic initiation and [18F]FDG-PET/CT performance (a median of 13 days for false negatives and 5 days for true positives, P=.19). Nonetheless, significance could be masked by the small number of cases. The absence of false positive results in our cohort can be partially explained by the longer period between device implantation and [18F]FDG-PET/CT acquisition in controls, which was a median of 6.1 [0.05-24.31] years. In the study by Jeronimo et al.,12 the median time between device implantation and [18F]FDG-PET/CT was 2.3 [0.6-6.4] years. That study, as well other published works,14,15 state that false positive results are caused by postoperative inflammatory activity.
Although TEE plays an essential role in the diagnosis of lead infection, it may be hard to differentiate vegetations from lead strands or small adhered thrombi.16 It is commonly accepted that TEE is initially performed in patients with suspected SI, whereas [18]FDG-PET/TC should be the primary technique to confirm LI due to the lower sensitivity of [18F]FDG-PET/CT for endovascular and intracardiac lead infections. Concordantly, in our cohort, TEE showed higher accuracy in diagnosing intracardiac lead infections. However, it is worth noting that the performance of [18F]FDG-PET/CT was better in subcutaneous and endovascular lead infections in SI cases with bacteremia. A negative TEE result does not rule out SI 12 and, considering that Pizzi et al. demonstrated an increased sensitivity of [18F]FDG-PET/CT in combination with TEE,17 our results suggest that [18F]FDG-PET/CT may not be the only the test of choice to confirm an active local infection15 but may also be complementary to TEE in SI cases. Our data showed that [18F]FDG-PET/CT used in combination with TEE significantly increased the rate of definite diagnosis of infection from 30.4% to 56.1% (P=.04) due to the detection of endovascular lead [18]FDG uptake. Furthermore, [18F]FDG-PET/CT has the additional value of being able to detect septic embolisms,14,18–20 as occurred in 2 of our SI cases. This datum seems to be consistent with that published by Rodríguez-Alfonso et al.,21 who showed that [18F]FDG-PET/CT correctly reclassified 57% of patients with initial suspicion of generator pocket infection by detecting lead infection with high diagnostic performance, especially in patients with initial suspicion of LI.
Some authors suggest that an increase in the metabolic rate of the spleen and BM could be used as an indirect sign of infection.4 Our study could not corroborate this hypothesis, as SUVmean spleen and SUVmean BM were similar in cases and controls and between LI and SI. However, most control cases were patients with cancer, in whom spleen and/or BM uptake could have been increased due to their neoplastic disease, chemotherapy, or other hematological alterations. Nonetheless, we found significative differences in spleen and BM metabolism between those patients with SI and confirmed bacteremia compared with LI cases. These results may be explained by the expected hyperactivation of the phagocytic mononuclear system in cases of bacteremia, which could be helpful in distinguishing bacteremic lead infections from isolated LI.
Complete device removal in CIED-IE is mandatory to cure infection4,22; however, in the last few decades a higher number of patients cannot undergo complete CIED extraction surgery,5 even if indicated, due to the growth in comorbidities, older age, and more complex infections. In these cases, CAS has been proposed as a helpful strategy. In our cohort, patients with incomplete device removal received undefined CAS, usually lifelong, which represented a heavy burden for patients and led to adverse effects, multidrug-resistant infections, and a high cost for the health system. To date there is no tool to guide clinicians on when to stop CAS. We studied 6 cases in which [18F]FDG-PET/CT, in combination with the clinical course and laboratory and microbiological findings usefully guided physicians in discontinuing CAS in the absence of relapse for more than 2 years of follow-up. Despite the limited number of cases in our cohort, this study supports the idea that further prospective studies could validate [18F]FDG-PET/CT as a reliable tool for discontinuing CAS safely during the follow-up of cases with incomplete device removal.23,24
LimitationsThis study has some limitations. First, it is a retrospective study with limitations on data interpretation; therefore, data on previous antibiotic therapy was not achieved for each case. Second, we were unable to evaluate intracardiac leads in the [18F]FDG-PET/CT scans of control participants, as they did not undergo a myocardial inhibition protocol. Therefore, we excluded the specificity analysis for the intracardiac lead. In addition, a high-fat, low-carbohydrate diet before [18F]FDG-PET/CT scanning was not systematically applied to all patients. Third, comparisons between BM and spleen uptake were based on small subgroups of patients with low statistical power. Fourth, device implantation was more longstanding in controls than in cases and therefore we were unable to assess the accuracy of [18F]FDG-PET/CT in recently implanted CIEDs. Finally, the number of cases in which CAS therapy was discontinued based on negative [18F]FDG-PET/CT scans was small and these preliminary results should be confirmed in further studies with a larger set of patients.
The key findings of this study are the high sensitivity and specificity of [18F]FDG-PET/CT for identifying LI and its unique role in the assessment of subcutaneous and endovascular lead infection, which cannot be evaluated by any other diagnostic techniques. This work is the first to compare spleen and BM metabolism and their potential usefulness in stratifying CIED infections, showing their potential role in detecting bacteremia. In addition, our cohort is the largest published case-control series and the only study evaluating [18F]FDG-PET/CT in the management of CAS therapy in patients with incomplete device removal.
CONCLUSIONSThe diagnostic performance of [18F]FDG-PET/CT is high in local CIED infections but lower in endovascular and intracardiac lead infections. However, [18F]FDG-PET/CT is the only available technique for assessing subcutaneous and endovascular lead infection and may be complementary to TEE in cases of bacteremia, increasing the definite diagnosis of lead infections. Moreover, spleen and BM metabolism may help to distinguish between bacteremic lead infections and isolated LI. Although further prospective studies are needed, follow-up [18F]FDG-PET/CT could potentially play a role in the management of CAS therapy when complete device removal is unachievable.
- –
[18F]FDG-PET/CT has improved the diagnosis of CIED infections and has been incorporated as a major diagnostic criterion in guidelines on prosthetic valve endocarditis.
- –
Although the diagnostic yield of [18F]FDG-PET/CT is high for the pocket, its accuracy in other CIED topographical regions requires better characterization.
- –
TEE is the gold standard for diagnosis but does not differentiate well between thrombus and vegetation. Many patients with bacteremia probably have endovascular lead infection, which TEE cannot detect.
- –
Hypermetabolism of the spleen and bone marrow detected by [18F]FDG-PET/CT has recently been shown to be an indirect sign of infective endocarditis in native or prosthetic valves.
- –
There are no data on the usefulness of [18F]FDG-PET/CT in guiding the duration of chronic oral antimicrobial therapy in patients with CIED infections without complete device removal.
- –
[18F]FDG-PET/CT has high overall specificity and sensitivity for local infections of the generator pocket but lower sensitivity in systemic infections and other topographical sections of the CIED lead.
- –
We demonstrate that [18F]FDG-PET/CT combined with TEE can significantly increase the rate of definite diagnosis in endovascular and intracardiac lead infections.
- –
Spleen and bone marrow hypermetabolism may help distinguish systemic bacteremia from isolated local CIED infections.
- –
When complete device removal is unachievable, a follow-up negative [18F]FDG-PET/CT might guide physicians in discontinuing suppressive oral antimicrobial therapy.
J. M. Miró has an Instituto de Investigaciones Biomédicas August Pi i Sunyer (IDIBAPS) personal intensification research grant from 2017 to 2023. MHM held a Rio Hortega Research Grant (CM17/00062) from 2018 to 2020. All other authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
AUTHORS’ CONTRIBUTIONAll the authors contributed to the conception and design, data acquisition, drafting of the article, critical revision, and final approval of the manuscript. The data underlying this article will be shared on reasonable request to the corresponding author (data available on request). M. Hernández-Meneses and A. Perissinotti contributed equally as first authors. The members of Hospital Clinic of Barcelona Infective endocarditis Team investigators are listed in the .
CONFLICT OF INTERESTSNone of the authors have any association that might pose a conflict of interest in this work. J.M. Miró, as the corresponding author, declares having no conflicts of interest. As a corresponding alternative author, D. Fuster claims to have no conflicts of interest.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2023.04.001




![Central illustration. The figure shows examples of positive FDG uptake and sensitivity values of [18F]FDG-PET/CT in a 3D visual representation of each CIED topographical region: pocket (blue), subcutaneous (green), endovascular (yellow), and intravascular (red). 3D, three-dimensional; [18F]FDG-PET/CT, 18F-fluorodeoxyglucose-positron emission tomography/computed tomography. CIED, cardiac implantable electronic-device. Central illustration. The figure shows examples of positive FDG uptake and sensitivity values of [18F]FDG-PET/CT in a 3D visual representation of each CIED topographical region: pocket (blue), subcutaneous (green), endovascular (yellow), and intravascular (red). 3D, three-dimensional; [18F]FDG-PET/CT, 18F-fluorodeoxyglucose-positron emission tomography/computed tomography. CIED, cardiac implantable electronic-device.](https://static.elsevier.es/multimedia/18855857/0000007600000012/v2_202401211422/S1885585723001019/v2_202401211422/en/main.assets/thumbnail/gr1.jpeg?xkr=eyJpdiI6ImZKZjY1RGtMaE1VWXRGQ2R1SWNiY3c9PSIsInZhbHVlIjoiTGc2TGFyOTczS2hnWFR1YlJJcWN0UlZZQy9vUWNsT1gyd3JkV2d0bHM3ND0iLCJtYWMiOiJkNGMyNWIxODg3OWFiY2U5Y2U5MGI1YzM0Njc1MDdkNGI2YmI0OGUxZjk2MGUyMTAyMzk1MDg1YzQ4ODBiY2I5IiwidGFnIjoiIn0=)
![A: ROC curve for CIED pocket SUVmax cutoff point 2.35 [sensitivity: 79.63%; 92.59%]. B: ROC curve for CIED pocket SUVmax/SUVmean liver, cutoff point 1.28 [sensitivity: 75.56%; specificity: 88.89%]. CIED, cardiac-implantable-electronic-device; ROC, SUV, standardized uptake value, SUVmax, maximum standardized uptake value. A: ROC curve for CIED pocket SUVmax cutoff point 2.35 [sensitivity: 79.63%; 92.59%]. B: ROC curve for CIED pocket SUVmax/SUVmean liver, cutoff point 1.28 [sensitivity: 75.56%; specificity: 88.89%]. CIED, cardiac-implantable-electronic-device; ROC, SUV, standardized uptake value, SUVmax, maximum standardized uptake value.](https://static.elsevier.es/multimedia/18855857/0000007600000012/v2_202401211422/S1885585723001019/v2_202401211422/en/main.assets/thumbnail/gr2.jpeg?xkr=eyJpdiI6IlJISDgyclB3Y011Z3F6R29tcFZ3Unc9PSIsInZhbHVlIjoicXJiVHhMVFR2QmMzVG1Jbm5XaE1VL2VlLzlacnJycWRKcnhTbDdVcGlUZz0iLCJtYWMiOiJkYTQ4OTkzZWUzNmYwYjNlMzQwODgxNmVlOWNlYWQ3ZDFiMGQ4NTFhMTVkYjA5ODZmNDk5ZDg3ZTAzOGUzNWQ2IiwidGFnIjoiIn0=)