A diagnosis of cardiac amyloidosis often requires histological evidence of amyloid deposits, either in the heart itself or in biopsies from other affected organs because prognosis and treatment vary considerably according to the type of amyloidosis.1 The appearance of global, subendocardial late gadolinium enhancement (LGE) has been described as highly characteristic of cardiac amyloidosis, and is associated with a ∼ 5-fold increase in mortality.2 However, ∼ 7% of the patients present with an atypical LGE pattern such as focal subendocardial or midmyocardial LGE for which the prognostic significance and therapeutic implications are unclear.3 Regression of cardiac light-chain (AL) amyloidosis has been reported following autologous stem cell transplant (ASCT) or chemotherapy in patients with the characteristic LGE pattern for cardiac amyloidosis.4,5 However, little is known about the impact of these therapies in patients with atypical patterns of LGE.
Here, we present 3 patients with AL amyloidosis with an atypical LGE pattern who underwent ASCT and serial imaging with cardiac magnetic resonance imaging (RMI) and transthoracic echocardiogram (TTE) before and after treatment (respectively 10, 21 and 23 months). TTE with global longitudinal strain (GLS) and RMI (1.5T) including cine-RMI, LGE, and T1-mapping were performed before and after ASCT. Left ventricular ejection fraction (LVEF), native T1-relaxation times, and extracellular volume (ECV) fraction were calculated from RMI images using commercially available software (qmass, Medis Medical Imaging systems, Leiden, Netherlands). GLS was calculated from TTE images using QLAB software (Phillip Medical Systems, Andover, Massachusetts). Clinical evaluation with electrocardiogram and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were also performed. Measurements from before ASCT were compared with those after ASCT using a t test.
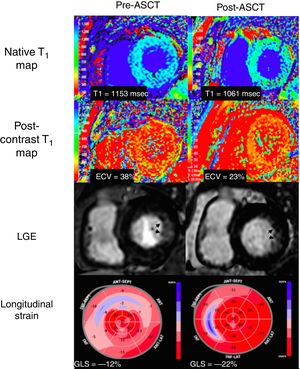
Patients were aged 63, 64 and 73 years and 1 was female. All patients had biopsy proven AL amyloidosis and underwent ASCT with complete hematological response. These patients were at low risk for ASCT according to the Mayo Clinic staging system. Native myocardial T1-relaxation time and ECV were increased at baseline and decreased following ASCT (Figure 1, T1: 1186±30 msec vs 1119±50 msec, P=.036 and ECV: 41±6% vs 28±10%, P=.026). LGE was present in 2 patients in the subendocardial inferior and inferolateral walls, and in 1 patient in the subendocardial anterolateral wall. LGE persisted in the post-ASCT MRI but was slightly improved following ASCT (Figure 2). An “apical sparing” pattern of longitudinal strain (ie, abnormal in the basal and mid levels of the left ventricle but relatively normal in the apical levels) was present in all cases. GLS was abnormal at baseline and improved significantly after ASCT (14±1% to−20±3%, P=.037), with statistically inconclusive changes in LVEF (53±8% vs 54±7%, P=.074). Left ventricular wall thickness, left atrial volume and NT-proBNP levels did not change significantly after ASCT. Two patients had typical electrocardiogram changes of amyloidosis (low voltages or pseudoinfarct pattern) before ASCT, which improved in 1 after transplant. All patients were alive after a median follow-up of 23±7 months and had good functional capacity.
In our case series, significant improvement in ECV, native myocardial T1-relaxation times and GLS were noted following ASCT in patients with atypical patterns of LGE, despite no significant changes in the more commonly used imaging-based biomarkers of cardiac amyloidosis, such as LVEF, left ventricular mass, or left atrial volume.
The most likely mechanism to explain this paradoxical behavior is probably differences in the inherent measurement variability of the different parameters. However, other possible explanations might include direct myocardial injury from residual light chain deposits in the myocardium the chemotherapeutic agents used to treat AL amyloidosis. Another possible reason paradoxical behavior might be observed is the development of arrhythmias such as atrial fibrillation or valvular lesions such as mitral regurgitation. A larger cohort of patients would be needed to better understand the underlying reason the various parameters are not uniformly aligned.
Increasing LGE (none, subendocardial, transmural) has been associated with structural and functional changes (eg, increased left ventricle mass, decreased LVEF, left atrial dilation) and worsening tissue characterization (elevated native myocardial T1-relaxation times and ECV).6
Previous research has shown that patients with typical LGE patterns of cardiac amyloidosis have higher left ventricular wall thickness, higher left ventricle mass, and worse diastolic function compared with those with atypical LGE.3 Interestingly, patients in our case series had impaired longitudinal strain in the basal segments that improved following ASCT, suggesting early myocardial involvement despite the absence of a characteristic global subendocardial LGE pattern.
Regarding NT-proBNP levels, 2 patients had lower levels after treatment and 1 had a small increase. NT-proBNP change is a marker of treatment response only if baseline NT-proBNP> 650 ng/L. In our series, only 1 patient had NT-proBNP levels above this limit and showed a significant decrease after treatment. Interestingly, the RMI and TTE parameters improve despite baseline NT-proBNP levels.
In conclusion, these data suggest that ASCT treatment for cardiac amyloidosis with an atypical LGE pattern can result in regression of the infiltrative process assessed by improvements in native myocardial T1-relaxation times, ECV, and GLS.