There is increasing recognition that coronary microvascular dysfunction also plays an important role in coronary heart disease. Little is known about this aspect of coronary heart disease due to difficulties in studying the coronary microcirculation directly. The retina is a unique site where the microcirculation can be imaged directly, providing an opportunity to study in vivo the structure and pathology of the human circulation and the possibility of detecting changes in microvasculature relating to the development of cardiovascular disease. This review covers the recent progress in research linking retinal vascular signs to coronary heart disease, and finds accumulating evidence that retinal vascular signs may provide a window into the health of the coronary microvasculature. The most widely studied signs, arteriolar narrowing, and more recently, venular dilation, are likely associated with increased risk of coronary heart disease in women, independent of traditional risk factors. Attempts to improve coronary heart disease risk prediction by incorporating retinal vessel caliber size into risk prediction scores complementing traditional algorithms such as the Framingham risk scores have so far been disappointing. Research is ongoing into the predictive utility of other retinal vascular signs. Retinal photography provides long-lasting records that enable monitoring of longitudinal changes in these retinal signs and vascular health.
Keywords
.
IntroductionCoronary heart disease (CHD) is the leading cause of death worldwide. While the majority of CHD is attributable to coronary artery disease in the epicardial coronary arteries, there is increasing recognition that coronary microvascular dysfunction also plays an important role in CHD.1, 2 Little is known about this aspect of CHD due to difficulties in studying the coronary microcirculation directly.
Role of small vessel disease in coronary heart diseaseThere is a subgroup of patients who present with angina-like chest pains, but when they undergo coronary catheterization and angiography are found to have minimal atherosclerotic plaques, a condition commonly known as cardiac syndrome X.1, 2 It is believed that this group of patients has coronary microvascular dysfunction, based on electrocardiographic evidence of ST-segment depression during spontaneous or stress-induced chest pain, as well as reversible stress-induced defects in myocardial perfusion.3 However, confirming the diagnosis of microvascular dysfunction is difficult due to the lack of noninvasive modalities to image the coronary microcirculation. The microvasculature changes that underlie angina attacks are also unclear, and may be related to focal ischemia in small myocardial regions caused by pre-arteriolar dysfunction.4 This phenomenon of syndrome X appears to occur more commonly in women and in persons with diabetes.1 Diabetes is known to have profound effects on the microvasculature, supporting a microvascular role in syndrome X.
Analogous evidence supporting a parallel role of microvascular disease in some angina cases comes from the role of microvascular disease in some subtypes of stroke. Lacunar stroke, accounting for a quarter of ischemic stroke cases,5, 6 is known from magnetic resonance imaging and autopsy studies to be a disease of small cerebral penetrating arteries, although exact underlying small vessel pathologies are still uncertain.7, 8, 9 Recently, there has been renewed interest in studying the microvascular aspects of acute stroke, in an attempt to better understand various causes of acute stroke and thus better target therapies and improve rehabilitation outcomes. It may be that cardiac syndrome X is the cardiac equivalent of lacunar stroke.
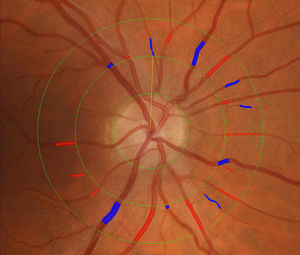
Retinal imaging and retinal vascular signsThe retina is a unique site where the microcirculation can be imaged directly, providing an opportunity to study in vivo the structure and pathology of the human circulation and the possibility of detecting changes in the microvasculature relating to the development of cardiovascular disease.10, 11, 12 Further, the retinal vasculature can be viewed directly not only by ophthalmoscopy but also photography, enabling lasting records over a series of time points. These photographic records can be magnified and studied in detail at a later time. Recent technological advances in high resolution digital photography and image processing software programs13, 14, 15 have enabled quantitative and reproducible measurement of various changes in the retinal vasculature, termed retinal vascular signs in this review. Figure 1 shows the application of an image software program to measure retinal arteriolar and venular caliber.
Figure 1. Measurement of retinal arteriolar and venular caliber using an image software program.
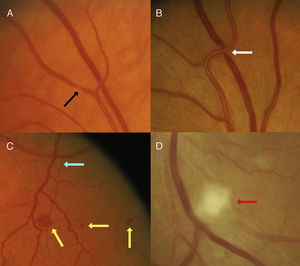
An important observation from initial studies is that various retinal vascular signs, including isolated microaneurysms and hemorrhages, focal arteriolar narrowing, and arterio-venous nicking, are relatively common in the adult population, and are detectable from retinal photographs in 2% to 14% of the nondiabetic population of adults aged over 40 years,16, 17, 18, 19 with new signs developing in 6% to 10% of people every 5 years.20, 21, 22 Figure 2 provides some examples of these signs, which if severe can be detected on dilated ophthalmoscopy.
Figure 2. Examples of retinal vascular signs. Black arrow: focal arteriolar narrowing. White arrow: arterio-venous nicking. Yellow arrow: haemorrhage. Blue arrow: microaneurysm. Red arrow: cotton wool spot.
Histopathological studies have demonstrated that these retinal signs reflect vascular damage from aging, hypertension, and other processes.12, 23, 24 Pathological studies have further suggested that retinal signs are closely related to microvascular pathologies of other organs (eg, in persons with hypertension, the retinal arteriole narrows and its media thickens and develops scleroses).12 Similar sclerotic changes have been observed in intramyocardial small arterioles, which in the presence of hypertension show luminal narrowing as in the retina.25, 26 Increased media to lumen ratio of arteries in subcutaneous fat independently predicts risk of cardiovascular disease events including myocardial infarction.27, 28 Biopsies of these subcutaneous small arteries (usually obtained from gluteal biopsies) indicate that vascular remodeling is one of the first manifestations of target organ damage, occurring before proteinuria or cardiac hypertrophy, and that it is a reversible, dynamic process.29, 30 Of clinical importance, the magnitude of remodeling of small arteries has prognostic significance over a 10-year period, with worse prognosis for hypertensive subjects with greater magnitude of remodeling.27 Arterioles have a similar structure to small arteries but less elastic and muscular fibers. The retinal vessels offer access to study these small arterial and arteriolar changes noninvasively.
Our group and other investigators have recently applied retinal microvascular imaging to study microvascular pathologies among acute stroke patients.31, 32 Findings from these studies showed a distinct range of retinal vascular signs more frequently associated with acute lacunar stroke compared to other ischemic stroke subtypes, supporting the view of predominantly localized arteriolar pathologies in the pathogenesis of lacunar stroke, and also suggesting a potential for retinal imaging to be used in studying small vessel disease.31, 32
Analogous to the link between the retina and the brain, there are indications that retinal vascular changes parallel pathological changes in both the coronary micro- and macro-circulation.33 In a study of 234 participants free from CHD, retinal arteriolar narrowing was strongly associated with reduced myocardial perfusion measures on cardiac magnetic resonance imaging.33 In other studies, retinopathy lesions were correlated with coronary artery calcification (measured on cardiac computed tomography scanning) in a dose response manner, with more severe lesions associated with worse coronary artery disease on angiography.34, 35 Thus there are suggestive anatomical, physiological and pathological reasons to believe that changes in the retinal microvasculature may be useful indicators of the vascular structural pathologies of the coronary micro-circulation,36 and that non-invasive retinal assessment may assist CHD risk stratification.36
Different Retinal Vascular Signs Are Associated With Different Coronary Heart Disease Risk FactorsA number of studies have reported that retinal vascular signs are associated with chronic elevation of blood pressure (BP)14, 37, 38, 39 and systemic markers of inflammation and endothelial dysfunction.39, 40, 41, 42 Studies have demonstrated that narrower retinal arterioles are strongly correlated with elevated ambient BP, and less strongly with prior BP levels.43 A consistent gradient of association between elevated BP and retinal arteriolar narrowing has also been shown in many studies.14, 37, 38, 39 In contrast, wider retinal venules may be a marker for cerebral hypoxia,44 endothelial dysfunction, hyperglycemia45 and inflammation.14, 39 Retinopathy lesions, meanwhile, have been associated with hyperglycemia, hypertension, endothelial dysfunction and inflammation.14, 46 Cumulative evidence from these previous studies implies that specific components of retinal vascular signs may convey information on different vascular disease processes and explain why some, but not all, retinal signs are associated with clinical CHD.47 It may be that retinal vascular signs such as arteriolar narrowing or venular widening are a summary marker of a patient's lifetime exposure to risk factors, and those with a predominantly hypertensive risk profile tend to have arteriolar narrowing while those with a risk profile of metabolic disorders tend to have venular dilation.
Retinal Vessel Caliber Predicts Risk of Coronary Heart DiseaseThe Atherosclerosis Risk in Communities (ARIC) study, a US cohort of over 10 000 individuals, was one of the first studies to quantitatively measure arteriolar and venular calibers, and reported that narrower arterioles, represented as lower arteriole to venule ratio (AVR), predicted 3-year risk of CHD events.48 This association was found only in women, not in men.48 Table 1 shows that women with narrower arterioles in the lowest two quintiles had two-fold higher risk of CHD, even after adjusting for traditional risk factors. This study was limited in that it was not clear if arteriolar narrowing or venular widening, or both, were responsible for the association of low AVR with incident CHD. The Blue Mountains Eye Study (BMES, n=3654) investigators sought to address this question by examining the association of caliber variations of both vessels with CHD mortality. This study reported that wider retinal venules predicted 9-year risk of CHD death in both men and women without a history of pre-existing CHD, while retinal arteriolar narrowing additionally predicted CHD death in women (Table 2).49 It should be noted, however, that the Beaver Dam Eye Study (BDES, n=4926) in the United States did not replicate these associations with 10-year all-cause or cardiovascular mortality.50 A pooled-data analysis from the BMES and BDES helped address this discrepancy. Retinal photographs in both studies were graded using standardized protocols. Only persons without CHD history were included in the analyses. Over a 10- to 12-year follow up period, both narrower retinal arterioles and wider retinal venules predicted 40% to 70% higher risk of CHD mortality in middle-aged persons (43 to 69 years)51 (Table 3), with weaker associations in those>70 years. These analyses were limited to CHD mortality outcome rather than CHD events.51 A case-control study from the BDES suggested other retinal signs such as retinopathy and focal arteriolar narrowing may also be associated with CHD mortality.52
Table 1. Retinal Arteriolar Narrowing and 3-Year Risk of Coronary Heart Disease.
| Retinal arteriole to venule ratio | Adjusted RR (95% CI) * | |
| Women | Men | |
| 1st quintile (range: 0.57-0.78) | 2.2 (1.0-4.6) | 1.1 (0.7-1.8) |
| 2nd quintile (range: 0.59-0.82) | 2.3 (1.1-4.8) | 1.0 (0.6-1.7) |
| 3rd quintile (range: 0.83-0.86) | 1.6 (0.8-3.4) | 1.2 (0.7-1.9) |
| 4th quintile (range: 0.87-0.91) | 1.3 (0.6-2.8) | 1.2 (0.7-2.1) |
| 5th quintile (range: 0.91-1.22) | 1.0 | 1.0 |
RR (95% CI): relative risk and 95% confidence interval (CI).
* Adjusted for age, race, field center, mean blood pressure, diabetes, cigarette smoking, alcohol consumption, waist-hip ratio, sport index, total and HDL cholesterol and triglycerides, and antihypertensive medication use.Source: Wong et al. 48 .
Table 2. Nine-Year Risk of Coronary Heart Disease Death in Persons ≤75 Years.
| No. at risk | RR (95% CI) * | |
| Women | ||
| Per SD decrease arteriolar caliber | 1565 | 1.9 (1.0-3.5) |
| Per SD increase venular caliber | 1564 | 2.0 (1.1-3.6) |
| Men | ||
| Per SD decrease arteriolar caliber | 1210 | 1.0 (0.7-1.6) |
| Per SD increase venular caliber | 1210 | 1.8 (1.1-2.7) |
RR (95% CI): relative risk and 95% confidence interval; SD: standard deviation.
* Adjusted for age, smoking, diabetes and systolic blood pressure, and arteriolar and venular caliber in the same model.Source: Wang et al. 49 .
Table 3. Retinal Vascular Signs and 10- to 12-Year Coronary Heart Disease Mortality.
| Retinal vascular sign | Study | Follow-up period (years) | Coronary Heart Disease MortalityAdjusted RR (95% CI) * |
| Arteriolar narrowing | Pooled BMES, BDES 51 (cohort studies) | 10-12 | 1.70 (1.27-2.28) |
| Venular dilation | Pooled BMES, BDES 51 (cohort studies) | 10-12 | 1.41 (1.06-1.89) |
| Retinopathy | BDES 52 (case-control) | 10 | 1.8 (1.2-2.7) |
| Focal narrowing | BDES 52 (case-control) | 10 | 2.7 (1.0-7.4) |
| Arterio-venous nicking | BDES 52 (case-control) | 10 | 1.8 (0.8-4.5) |
BDES, Beaver Dam Eye Study; BMES, Blue Mountains Eye Study; RR (95% CI), relative risk and 95% confidence interval.
* Adjusted for age, sex, smoking, hypertension, diabetes, HDL-C and other risk factors. Arteriolar narrowing defined as measurements within the narrowest quintile; venular dilation as measurements within the widest quintile, with other quintiles as the reference group.
The most recent evidence supporting that retinal vessel caliber changes predict CHD comes from a meta analysis of 21 428 individuals, in whom 2076 (9.7%) incident CHD events were recorded.53 These individuals had a mean age of 62 years, all were free of CHD at baseline, and were followed for 5 to 14 years. Analyses adjusted for traditional cardiovascular risk factors. The meta-analysis found that both narrower retinal arterioles and wider retinal venules were associated with an increased risk of CHD in women but not in men. In women, the pooled multivariable-adjusted hazard ratios (HR) were 1.19 (95% confidence intervals [CI] 1.09 to 1.30) per 20μm decrease in arteriolar caliber and 1.18 (95% CI 1.08 to 1.29) per 20μm increase in venular caliber. In men, the corresponding results were HRs of 1.05 (95% CI 0.97 to 1.14) per 20μm decrease in arteriolar caliber and 1.02 (95% CI 0.95 to 1.11) per 20μm increase in venular caliber. Higher HRs were found amongst women without hypertension or diabetes.53 These results are consistent with the concept that coronary microvascular disease may play a greater role in women than in men,54, 55, 56 and may account for sex-based differences in CHD presentation (women with chest pain frequently show nonobstructive coronary angiograms), and in outcomes with revascularization or bypass grafting (worse in women).26, 27, 28, 57 Compared to men, women have smaller coronary arteries with more diffuse atherosclerosis and more severely impaired arteriolar vasodilator responses.54 Arteriolar narrowing in response to aging, elevated BP and endothelial dysfunction may further compromise myocardial perfusion, leading to increased CHD risk in women.48, 55 The pathophysiological implications of wider retinal venules in predicting increased CHD risk in women is unclear at present but is consistent with reported associations of this retinal vessel change with inflammatory markers, endothelial dysfunction and increased aortic and large arterial wall stiffness.58, 59, 60
The finding that retinal vessel caliber independently predicts risk of CHD has led to suggestions that retinal photography and measurement of vessel caliber may help CHD risk stratification. To explore this idea, investigators have analyzed data from the ARIC study to see if incorporating retinal vessel caliber into Framingham risk models improves risk prediction in women.61 Area under the receiver operator characteristic curve was used as an indicator of improved prediction, and the investigators found that it increased only from 0.695 to 0.706 (1.7% increase) with the addition of retinal vascular caliber to the Framingham risk model. The conclusion drawn was that the incremental predictive ability of retinal vessels over that of the Framingham model was very modest, and unlikely to influence clinical practice or clinical outcomes meaningfully.61
Other Retinal Vascular Signs and Coronary Heart DiseaseThere is evidence that other retinal vascular signs, in addition to retinal vessel caliber, may also predict CHD, but these other signs have not been investigated in detail. The BDES investigators found that other retinal arteriolar signs such as focal narrowing, arteriovenous nicking, and retinopathy (examples shown in Figure 2) were predictive of higher risk of CHD mortality in the BDES population (Table 3). However, these findings have not been confirmed in other studies.50 In persons with diabetes it is well known that retinopathy lesions signal increased cardiovascular risk.62, 63, 64, 65 The ARIC study reported that in persons with type 2 diabetes, the presence of retinopathy lesions was associated with a two-fold higher risk of incident CHD and three-fold higher risk of fatal CHD, independent of glycemia levels, cardiovascular risk factors and large vessel atherosclerosis.62 The association showed a gradient pattern with increasing retinopathy severity, and was significant in both men (HR 1.89, 95% CI 1.08 to 3.31) and women (HR 2.16, 95% CI 1.16 to 4.02), and in persons without hypertension.62 The BMES reported that retinopathy lesions were associated with CHD mortality both in persons with and without diabetes.66 Further, the additional increased CHD risk associated with retinopathy in persons without diabetes was similar in magnitude to the risk associated with the presence of diabetes alone. 66 Similar findings were reported from the Hoorn Study67, which was the only other study examining the association of retinopathy with CHD in persons without diabetes.
Retinal vein occlusion (RVO) is an uncommon condition, but due to its association with cardiovascular risk factors, particularly hypertension, it may be an independent predictor of CHD. This question has been addressed by a pooled study of the BMES and BDES.68 This pooled analysis studied 8384 baseline participants, of whom 96 (1.14%) had RVO at baseline (BDES, n=38; BMES, n=58). After follow-up of 12 years, 1312 (15.7%) died of cardiovascular-related conditions. After adjusting for age, sex, body mass index, hypertension, diabetes, smoking, glaucoma, and study site, RVO was not associated with cardiovascular-related mortality (HR 1.2, 95% CI 0.8 to 1.8) among participants of all ages. However, in persons aged less than 70 years, baseline RVO was associated with higher cardiovascular mortality (HR 2.5, 95% CI 1.2 to 5.2). 68 This result should be balanced with findings from a case control study that recruited 329 patients with RVO and compared their mortality with that of the general population, and found no difference in all cause mortality rates.69
Retinal emboli often originate from a cardiac or carotid artery plaque, and whether they predict incident CHD mortality has been studied in the BDES.70 This study found that the 10-year cumulative incidence of retinal emboli was 1.5%, and that retinal emboli were strongly associated with a history of coronary artery bypass surgery (odds ratio [OR] 7.17, 95% CI 3.18 to 16.18).70 However, retinal emboli at baseline did not predict CHD mortality over a 10-year period. Results from the BDES were pooled with BMES results, and the combined analysis again found no association between retinal emboli, detected at baseline, and CHD mortality (HR 1.2, 95% CI 0.8 to 1.7), although a strong association with stroke mortality was evident (HR 2.0, 95% CI 1.1 to 3.8).71 One group examined whether coronary catheterization causes retinal embolisation in 97 patients attending for coronary catheterization.72 Before catheterization, retinal emboli were observed in 5 patients (5.2%) and no new emboli were found within the 16-hour (median; with a range of 4 to 45h) post-coronary catheterization period. The presence of angiographic coronary artery disease was not significantly associated with pre-existing retinal emboli, and the authors reported no evidence suggesting that coronary catheterization contributes to retinal embolism in the short term. Nonetheless, another similar study found a 2% risk of acute retinal embolism within 3h post cardiac catheterization.73
Cardiovascular medications and the eyeSome cardiovascular medications such as amiodarone are known to have ocular side effects. Corneal verticillata, or vortex keratopathy presenting as superficial corneal whorls, is almost universal.74 These changes are reversible and usually of no clinical consequence. Other side effects include anterior subcapsular lens opacities and optic neuropathy. Amiodarone-induced optic neuropathy is uncommon and not believed to be dose-related.74 Some patients present with reduction in vision which may be bilateral, most commonly within 12 months of commencing therapy. Visual field defects are often present, as is bilateral optic disc swelling. Symptoms and signs may or may not be reversible on cessation of therapy. Patients on amiodarone who report visual disturbances, particularly within the first year of starting treatment should be referred for ophthalmological review.
Some ocular medications may have cardiovascular effects, such as timolol 0.5%, which is widely used in the treatment of glaucoma. Systemic absorption of topical timolol occurs and is known to result in reduction of heart rate, although without any changes in BP.75 This is believed to be due to compensatory increases in peripheral resistance. A report from the BMES found an increased risk of cardiovascular mortality in those taking timolol eye drops, although this may be related to confounding effects from other cardiovascular risk factors.76, 77 Reduction of systemic absorption can be achieved through instructing patients to apply firm pressure to the lacrimal puncta for a few minutes after instilling the eye drops.
Limitations of data and further researchMuch of the evidence reported above comes from large, well designed population-based studies with good measures on retinal signs and CHD outcomes. However, it is important to note that the majority of studies have reported only associations with CHD outcomes without direct angiographic evidence of coronary artery disease or coronary microvascular dysfunction. It therefore remains somewhat speculative if retinal microvascular changes do indeed mirror similar coronary microvascular changes. It is also not clear if these changes occur concurrently in multiple end organs or only in one target organ, such as the cerebral or coronary microvasculature, where changes are more correlated to retinal microvasculature than other end organs. Finally, no prospective studies to date have yet examined if retinal microvascular signs predict CHD recurrent events or mortality in persons with pre-existing CHD, a group in whom the risk of CHD is greatest.
Clinical implicationsThis review finds accumulating evidence that retinal vascular signs may provide a window into the health of the coronary microvasculature. The most widely studied signs, arteriolar narrowing and more recently venular dilation, are likely associated with increased risk of CHD in women, independent of traditional risk factors. Attempts to improve CHD risk prediction via incorporating retinal vessel measurements into risk prediction scores complementing traditional algorithms such as the Framingham risk scores have so far been disappointing.61 Research is ongoing into the predictive utility of other retinal signs. Another speculative potential application of retinal imaging may be used as a novel method to improve identification and diagnosis precision of cardiac syndrome X. There is a lack of studies examining this potential usage and this field warrants further investigation.
How should current information and retinal imaging be translated into clinical usage? A recent review has recommended an updated classification system of these retinal signs, which, because of their close association with hypertension, are often referred to as hypertensive retinopathy.78 This new classification system divides hypertensive retinopathy into 4 levels: none; mild, which refers to the presence of generalized and focal arteriolar narrowing, and arteriovenous nipping; moderate, which refers to the presence of lesions such as microaneurysms and hemorrhages, hard and soft exudates (cotton wool spots) (Figure 2); and severe, referring to optic disc edema. The authors recommend physicians undertake more vigilant monitoring of cardiovascular risk profiles in patients with mild retinopathy and adopt a more aggressive approach to risk reduction in patients with moderate retinopathy, while optic disc swelling requires urgent intervention to lower BP. The presence of these signs could be elicited either through ophthalmoscopy or photography after pupil dilatation. Patients of ophthalmologists and optometrists often have such photographs taken digitally, which are better records than ophthalmoscopic examination and enable monitoring of longitudinal changes in these retinal signs as well as in vascular health.
FundingSupported by the Australian National Health & Medical Research Council (NHMRC), Canberra, Australia (Project Grants IDs 153948 and 302068, and NHMRC Senior Research Fellowship to J. J. Wang).
Conflicts of interestNone declared.
Received 14 February 2011
Accepted 20 February 2011
Corresponding author: Centre for Vision Research, Department of Ophthalmology and Westmead Millennium Institute, University of Sydney C24, Westmead Hospital, NSW 2145, Australia. jiejin_wang@wmi.usyd.edu.au