Transcatheter aortic valve implantation (TAVI) has become the treatment of choice for most patients with severe symptomatic aortic stenosis. The European Society of Cardiology guidelines1 recommend with a level of evidence based on expert consensus (I-C) that TAVI only be performed in hospitals with on-site cardiac surgery. However, more and more clinical data indicate the value of a different level of recommendation on this topic, one with a scientific basis.
In this regard, data were recently published from a European registry (EuRECS-TAVI)2 of patients who required emergency cardiac surgery during transfemoral TAVI. Of the 27 760 patients included, 212 (0.76%) required emergency cardiac surgery; this figure has remained stable since 2014. The most frequent reasons for the emergency surgery were left ventricular perforation and annular rupture, which together occurred in half of the population. At 1 year of follow-up, all-cause mortality was high, even in patients who underwent emergency surgery and who were discharged alive (60%).
In 2014, a substudy of the German TAVI registry3 was published that compared clinical results between patients who had been treated in hospitals with and without on-site cardiac surgery. In total, 1432 patients were included; 12% (n=172) underwent TAVI in hospitals without on-site cardiac surgery. Their baseline characteristics were similar (logistic EuroSCORE, 20±11 in centers without on-site surgery and 21±14 in centers with on-site surgery), although the patients treated in centers without on-site surgery were hemodynamically more stable and more frequently had a history of cardiac surgery. Regardless of procedure duration, the complication rates were similar. In the Austrian TAVI registry,4 290 patients (15.9%) with high surgical risk who underwent transfemoral TAVI in centers without on-site cardiac surgery were compared with 1532 (84.1%) treated in centers with on-site cardiac surgery. The patients treated in hospitals without on-site cardiac surgery had a significantly worse risk profile: surgical risk before matching, 20.9 (12.8-30.3) in centers without on-site cardiac surgery vs 14.2 (9.0-22.2) in centers with on-site cardiac surgery. However, after matching, the risk score of the surgery group was 19.6 (13.1-28.6). After propensity score analysis, the short- and long-term mortality rates were similar in the 2 groups.
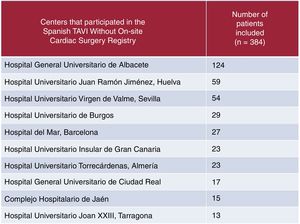
In Spain, patients have undergone TAVI in centers without on-site cardiac surgery since 2010. All of these centers use self-expanding prostheses and have on-site cardiovascular surgery and an arrangement with a cardiosurgical center that would accept urgent patients if required. The clinical results of these centers without on-site cardiac surgery in Spain were recently published.5 This registry is the largest to date (n=384 patients) (figure 1). The patients had moderate-to-high risk (mean STS, 5.9±3.7) but were older and had a higher prevalence of frailty than those in other registries. In this study, all implanted prostheses were self-expanding, conversion to surgery occurred in 1 patient (0.3%), and in-hospital, 30-day, and 1-year mortality rates were 5.2%, 6.1%, and 12.2%, respectively.
In light of the good clinical results of the registries of patients treated in centers without on-site cardiac surgery and the potential advantages of TAVI in these centers, such as the absence of need to transfer unstable patients and a beneficial impact on waiting lists, we can conclude that TAVI performance in centers without on-site cardiac surgery, particularly with self-expanding prostheses, is a viable and reasonable option for selected patients, specifically inoperable patients and those with high surgical risk, advanced age, or frailty.6 Although these data should be confirmed in studies with a larger number of patients, we consider that, given the scientific evidence, the level of recommendation on this topic should be reviewed in the clinical practice guidelines.
CONFLICTS OF INTERESTR. Moreno has participated in and received payments for lectures and consultations and support to attend conferences from Edwards Lifesciences; is proctor for Lotus and Acurate Neo valves, both from Boston Scientific; has participated in lectures and consultations and received support to attend conferences from Boston Scientific; and is proctor for the Allegra valve from New Vascular Therapy. M. Pan has participated in and received payments for lectures from Abbott, Terumo Medical Corporation, and Philips Volcano. A. Pérez de Prado has participated in and received payments for consultations from Boston Scientific and iVascular SL and for lectures from Abbott, Braun Surgical, Terumo Medical Corporation, and Philips Volcano. P. Jiménez Quevedo has no conflicts of interest.
.