We have read with great interest the publication by Lezcano Gort et al.1 The authors have kindly reported their experience in which a 40-year-old postpartum woman with no relevant coronary risk factors was admitted for non–ST-segment elevation myocardial infarction in which optical coherence tomography and intravascular ultrasound images showed a multivessel spontaneous coronary artery dissection (SCAD).
In this regard, we would like to mention further considerations: SCAD is a misdiagnosed entity because of the difficulty of recognizing the pathognomonic multiple radiolucent lumen with contrast wall staining. This typical sign is absent in > 70% of SCAD cases and only intravascular imaging can help to verify arterial wall integrity.2,3
SCAD can manifest with several clinical presentations, including angina pectoris, any type of acute coronary syndrome, and cardiogenic shock or sudden cardiac death,4,5 causing a dramatic exitus considering the young age of most patients.
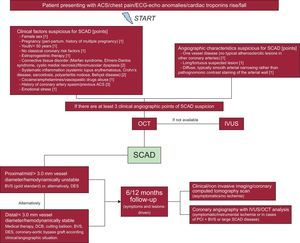
With these premises, we have recently published a flowchart (Figure6) for a faster diagnosis and proper treatment according to a literature review2–7 and our own experience.8–10
Flowchart for the diagnosis and management of SCAD. Reproduced with permission from Buccheri et al.6 ACS, acute coronary syndrome; BVS, bioresorbable vascular scaffold; DCB, drug-coated balloons; DES, drug-eluting stent; ECG, electrocardiogram; IVUS, intravascular ultrasound; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection.
Our flowchart assigns each clinical/angiographic risk factor for SCAD a score of up to 3 (Figure6). In a patient presenting with chest pain, ECG anomalies (ie, transitory/permanent ST-segment elevation), abnormal kinesis on echocardiogram, or cardiac troponin rise/fall with at least 3 points, according our score, we suggest the performance of an optical coherence tomography/intravascular ultrasound analysis for SCAD exclusion.
When we compared our point system with the case reported by Lezcano Gort et al.,1 we found more than 3 clinical points and very clear signs on coronary angiography (which add other 3 points).
Apropos the treatment strategy, we strongly greatly with the solution of Lezcano Gort et al.1 This treatment strategy is fully in accordance with that suggested in our flowchart. In fact, for asymptomatic patients with distal vessel SCAD or < 3.0 mm vessel diameter, we propose considering conservative management (first choice) or the use of one of the following devices: bioresorbable vascular scaffold, drug-eluting stent or drug-coated balloons, according to the clinical/angiographic characteristics of the patient.6,10
On the other hand, a bioresorbable vascular scaffold strategy should be preferred in cases of proximal/middle vessel lesion, ≥ 3.0 mm diameter or if the patient is still symptomatic/hemodynamically unstable, as reported by our group8,10,11and in line with an emblematic case previously published in this journal.12
Close follow-up with or without invasive coronary imaging to assess the risk of SCAD recurrence and the optimal sealing of the vessel over time is of primary importance.3,4
In conclusion, we wish to stress that a clinical/angiographic point system seems to be mainly useful in helping interventionists to avoid a missed diagnosis of SCAD. Furthermore, we believe that our treatment suggestion could be a good starting point in the absence of universal expert consensus or broad clinical experience to establish the most appropriate treatment for patients with SCAD.