Cardiovascular diseases are the principal cause of death in most economically developed countries, including Spain, and cause significant loss of health.1 Cost-effectiveness analysis is an important tool that can help clinicians, researchers, and policy makers to determine the efficiency of health care interventions, establish financing priorities for health services, and evaluate the effectiveness of these services in terms of health benefits and costs.2 The information provided by cost-effectiveness analysis thus has the potential to impact public health. Therefore rigor in clinical practice and health care policy requires careful evaluation of methods and results reporting in cost-effectiveness analyses to establish their validity. Previous studies systematically evaluated the methodology and general results of cost-effectiveness analyses that express their results as the cost per quality-adjusted life year (QALY) gained.3,4 However, to date, there has been no sufficiently detailed cost-effectiveness analysis of cardiovascular interventions in Spain. Such an analysis could provide comprehensive information about the state of research at a national level; the completeness of information reporting at this level is generally less well understood, even though specific health care priorities and research requirements are often established nationally. Here, we examine the quality of methods and results reporting in cost-effectiveness analyses of cardiovascular interventions in Spain.
Source data for this analysis were obtained from a previous systematic literature review of cost-effectiveness analyses of health care interventions published in Spain between 1989 and 2014.4 This literature review was conducted in PubMed and complementary databases (Scopus, ISI Web of Science, databases from the University of York Centre for Reviews and Dissemination, Índice Médico Español, Índice Bibliográfico Español en Ciencias de la Salud, and technology evaluation reports). From this review, we identified cost-effectiveness analyses of cardiovascular interventions that used QALYs as an outcome measure carried out in Spain up until December 2014. Based on existing documents and the review team's experience,4 we identified the basic elements of correct methods reporting in cost-effectiveness analyses. Data from each study were extracted by 2 reviewers. All data analysis was conducted with STATA v. 13 (StataCorp LP; College Station, Texas, United States).
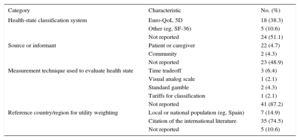
In total, 47 cost-effectiveness analyses were included. The studies are grouped by publication year in the of the and the main study characteristics are summarized in the of the . Most studies (n = 45 [95.7%]) did not indicate the research protocol used or provide access to it; moreover, most studies (n = 43 [91.5%]) used mathematical simulations. Fewer than half the analyses (n = 21 [44.7%]) provided a suitable description of the population characteristics. The interventions examined in most analyses (n = 30 [63.8%]) were classified as pharmacological therapies, and half the analyses (n = 24 [51.1%]) included an active alternative as the comparator. Data on intervention efficacy came from a single study of 21 analyses (44.7%), and only 9 analyses (19.1%) used synthetic estimates derived from systematic reviews and meta-analyses,5 even though such evidence is considered to be of high quality and scientifically rigorous. The methods used to calculate QALYs gained are shown in the Table. Few analyses (n = 5 [10.6%]) presented a complete description of the methods used to calculate QALYs. Most of the reports (n = 31 [66.0%]) stated that the evaluated intervention produced “more cost and more QALYs” than the comparator. Most of the analyses (n = 42 [89.4%]) reported favorable results. The main source of funding was the private sector, which supported 27 (57.4%) of the analyses. In 17 studies (36.2%) there was no declared conflict of interest.
Descriptive and Reporting Characteristics of Methods Used in Calculating QALYs in Cost-effectiveness Analyses of Cardiovascular Interventions in Spain
| Category | Characteristic | No. (%) |
|---|---|---|
| Health-state classification system | Euro-QoL 5D | 18 (38.3) |
| Other (eg, SF-36) | 5 (10.6) | |
| Not reported | 24 (51.1) | |
| Source or informant | Patient or caregiver | 22 (4.7) |
| Community | 2 (4.3) | |
| Not reported | 23 (48.9) | |
| Measurement technique used to evaluate health state | Time tradeoff | 3 (6.4) |
| Visual analog scale | 1 (2.1) | |
| Standard gamble | 2 (4.3) | |
| Tariffs for classification | 1 (2.1) | |
| Not reported | 41 (87.2) | |
| Reference country/region for utility weighting | Local or national population (eg, Spain) | 7 (14.9) |
| Citation of the international literature | 35 (74.5) | |
| Not reported | 5 (10.6) |
QALY, quality-adjusted life years.
The results presented here indicate room for improvement in the reporting of important features of cost-effectiveness analyses for cardiovascular interventions. Without complete and transparent information about how studies were performed, it is difficult to evaluate the validity of their results or the relevance of their conclusions. This concern has been addressed through the proposal of publication guidelines for cost-effectiveness analyses.6 These tools promote consistent reporting of a minimal information set that can serve as a benchmark for researchers carrying out these studies and for the reviewers and editors tasked with evaluating them for publication. The introduction of these guidelines into the peer review process could improve the quality of published cost-effectiveness analyses on cardiology. Authors, reviewers, and editors can promote complete reporting in cost-effectiveness analysis by using and promoting these guidelines as an important way to improve the transparency and completeness of published results.
FundingF. Catalá-López is supported by the Generalitat Valenciana (PROMETEOII/2015/021). M. Ridao has received funding from the Red de Investigación en Servicios de Salud en Enfermedades Crónicas (REDISSEC) of the Instituto de Salud Carlos III.