Patients with clinically evident coronary artery disease differ in their rate of progression, which impacts prognosis. We aimed to characterize serum and genetic markers in patients with rapid clinical progression (RCP) of coronary artery disease vs those with long standing stable (LSS) disease.
MethodsRetrospective study of cases (RCP) and controls (LSS) (1:2). Patients requiring ≥ 2 revascularizations due to atherosclerotic progression in the 10 years after a first angioplasty were considered to be RCP and those without events during the same period after the first angioplasty were considered to have LSS disease. After patient selection, we analyzed serum values, mRNA expression and genetic polymorphisms of inflammatory markers, including interleukin-6, C-reactive protein, and tumor necrosis factor (TNF)-a, and atherogenic markers consisted of proprotein convertase subtilisin/kexin type 9 (PCSK9), low-density lipoprotein receptor, sterol regulatory element binding transcription factor 2, and apolipoprotein-B.
ResultsThe study included 180 patients (58 RCP and 122 LSS). Demographic characteristics, classic risk factors and the extent of coronary disease were similar in the 2 groups. Patients with RCP showed higher serum levels of interleukin-6 and PCSK9 and higher TNF mRNA expression. Interleukin-6 rs180075C, TNF rs3093664 non-G and PCSK9 rs2483205 T alleles conferred a risk of RCP (P<.05 in all cases). Among patients with RCP, 51.7% had all 3 risk alleles vs 18% of those with LSS (P<.001).
ConclusionsWe suggest the existence of specific phenotypic and genotypic markers associated with RCP of coronary artery disease that could help to individualize the type and intensity of treatment.
Keywords
Coronary atherosclerosis is a chronic disease whose clinical presentation, ischemic heart disease, is a leading cause of death worldwide.
Patients with clinically evident coronary atherosclerosis differ widely in the rate of progression, with progression being one of the most important factors influencing prognosis.1,2 However, the ability to predict the risk of progression in a given patient is very limited. It would be highly valuable to be able to estimate progression risk to individualize the type and intensity of secondary prevention and treatment interventions.
Although there is no universal definition for progression of atherosclerosis, most studies describing this phenomenon have used an angiographic criterion: at least 10% reduction in the diameter of at least 1 pre-existing lesion of ≥ 50%, or ≥ 30% reduction in the diameter of a pre-existing lesion<50%, or progression of a lesion to total occlusion in a short period.3–8 However, this is an “anatomic” definition, not necessarily linked to clinical events.
The rapid progression scenario involves both anatomic-physiological phenomena (role of complex lesions) and inflammatory markers.4–6,9–12 Multiple studies have shown that inflammation is involved in the genesis, progression and instability of atherosclerotic plaque and leads the way to clinical syndromes.13–15 Accelerated progression of atherosclerosis has also been seen in patients with systemic inflammatory diseases such as rheumatoid arthritis.16 The role of lipid-related molecules has also been widely studied.17–29
To our knowledge, the concept of rapid progression of coronary atherosclerosis has always been based on the above-mentioned angiographic criteria, but we have found no reference in the literature to rapid clinical progression (RCP).
We believe that this is a limitation as progression in anatomical terms (on angiography or intravascular imaging) is much more sensitive but it is nevertheless a surrogate for what is really important: the risk of clinical recurrence. Therefore, a definition based on recurrence of clinical events could be much more valid.
Therefore, this study aimed to define “rapid clinical progressors” and analyze the phenomenon from a global perspective focusing on molecular substrates, mostly inflammatory markers and lipid molecules. We decided not to analyze thrombotic molecules as we aimed to focus our study on a stable phase of the disease and look for underlying factors that are present throughout the disease.
METHODSThe RAPROMS study (Rapid clinical progressor patient as an emerging clinical entity in patients with coronary atherosclerosis. Exploratory study on possible molecular substrates) is a hospital based single-center retrospective case-control study designed to assess and compare the molecular pattern of various agents involved in either the inflammation pathway or the metabolism of lipoproteins in 2 groups of patients: those with RCP of coronary atherosclerosis (case group) and those with long standing stable (LSS) disease (control group).
Definitions of population and subgroupsPatients with RCP were considered to be those fulfilling the following criteria: a) living patients with at least 1 coronary artery lesion treated with a percutaneous coronary intervention (PCI), and b) with at least 2 more PCIs in the following 10 years after the index PCI, due to disease progression (excluded restenosis) confirmed on angiography.
For the control group, LSS participants must fulfill all of the following criteria: a) living patients with at least 1 coronary artery lesion treated with a PCI, b) uneventful for cardiac events in a 10-year period after the index PCI, clinically asymptomatic for angina throughout that period, and with negative noninvasive tests for ischemia.
Patient selectionFor patient selection, a retrospective search was performed of the entire database of coronary angiograms in our center. Starting in 2007, we reviewed both consecutively and retrospectively all patients undergoing PCI, gathering information on new revascularization procedures or relevant clinical events in those patients in the 10 following years. Our hospital is the only tertiary referral hospital for PCI in the region. To gain power for the study, and assuming the RCP group would be smaller, we chose a 1:2 (case:control) design. LSS control participants were matched to RCP cases by age and sex.
Once the 2 groups of patients were identified, they were contacted, invited to participate, and, those who consented, signed the consent form and were enrolled in the study.
This study was designed, implemented and notified according to the ethical principles established in the Declaration of Helsinki. The protocol was approved by our local Independent Ethics Committee (Comité de Ética de la Investigación con Medicamentos de Cantabria), and a copy of all the consent forms remains properly filed.
Study proceduresClinical dataAn electronic case report form was designed to collect data on each patient's clinical status at the first revascularization event (index episode) and throughout the following 10 years, collecting all cardiovascular events experienced during that period regardless of the need for new revascularization. Information on traditional cardiovascular risk factors was collected at the time of the index episode, as well as the occurrence of new risk factors in the subsequent years and their adequate or inadequate control thereafter.
All information available on the treatment prescribed to each participant during the study period was collected in the most comprehensive manner possible, with special emphasis on treatment related to ischemic heart disease to detect potential patients without optimal standard treatment (due to lack of medical prescription or poor patient adherence). All treatment changes made during the 10-year period were noted.
Information was collected on the clinical presentation of each event leading to a new revascularization, the coronary anatomy in each procedure (SYNTAX score and morphology of the lesion), and the interventional treatment performed.
After signing the informed consent form, patients enrolled in the study completed a sociodemographic questionnaire and a blood sample was taken. No other action was required from participants.
The questionnaire was administered to participants with the aim of analyzing demographic and cultural, social and health aspects that might affect the course of the disease.
Laboratory analysisBlood samples were obtained from recruited patients at a stable stage (patients in the RCP group at least 6 months after the last event). After a deep literature review, we decided to analyze different inflammation/atherosclerosis markers at 3 different levels (protein levels, mRNA expression, and genetic polymorphisms) to allow a more complete approach to the disease. We selected interleukin-6, C-reactive protein (CRP) and tumor necrosis factor (TNF)-α as inflammatory markers and proprotein convertase subtilisin/kexin type 9 (PCSK9), low-density lipoproteins receptor (LDL-R), sterol regulatory element binding transcription factor-2 (SREBP2) and apolipoprotein (Apo)-B for the atherogenic perspective. A complete lipid profile was also obtained. A more detailed explanation of the techniques used for analysis can be found in Methods of the supplementary data. A list of the single nucleotide polymorphisms (SNP) selected in this study is provided in table 1 of the supplementary data.
Statistical analysisPrevious studies evaluating angiographically defined rapid progression of coronary atherosclerosis aimed to identify its association with various inflammatory or other markers included between 77 and 153 patients.3–8,30 For that reason, for our study, which evaluated inflammatory and lipid metabolism markers at various -omics, a sample of at least 160 patients was estimated, with a 1:2 ratio between the RCP and LSS groups.
Continuous variables are expressed as mean±standard deviation. The median [interquartile range] were obtained for all nonnormally distributed variables. The parametric or nonparametric distribution of the variables tested was analyzed with the Kolmogorov-Smirnov test. Categorical variables are expressed as percentages. Specific statistical analyses used for laboratory results can be found in the Methods section of the supplementary data. The strength of the associations was estimated by odds ratios (OR) and 95% confidence intervals (95%CI), and the results were adjusted by age, sex, diabetes mellitus, smoking, and hypertension using linear regression.
Statistical significance was set at P<.05. All statistical analyses were performed with SPSS 24.0. (Statistical Package for Social Sciences, United States) software and the STATA statistical software 12/SE (Stata Corp, College Station, United States).
RESULTSFrom the entire database of coronary angiograms in our site, we identified the first 70 patients who fulfilled the criteria for RCP, 61 of whom accepted to participate and signed the informed consent form. We matched these patients to 122 LSS participants. Finally, we were able to process 58 samples from the RCP group and all of the samples from the LSS group (figure 1 of the supplementary data).
Due to the location of the hospital where the study took place, all patients belonged to the same ethnic group (Caucasian).
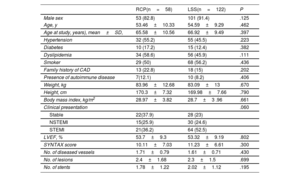
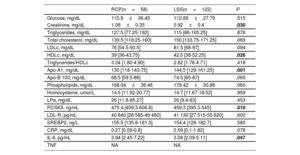
Demographic characteristics, risk factors profile and extent of coronary artery disease were similar in the 2 both groups. ST-elevation myocardial infarction as clinical presentation tended to be less frequent in the RCP group (36.2% vs 52.5%; P=.06) as shown in table 1. Table 2 shows serum levels of markers of inflammation/atherosclerosis and laboratory analytes in patients during a stable phase of their disease. Creatinine was significantly higher in the RCP group. Conversely, high-density lipoprotein and apo-A1 were significantly lower in the RCP group; low-density lipoprotein cholesterol also tended to be lower in this group.
Demographic characteristics, risk factor profile, clinical presentation and extent of coronary artery disease at first event in all patients
| RCP(n=58) | LSS(n=122) | P | |
|---|---|---|---|
| Male sex | 53 (82.8) | 101 (91.4) | .125 |
| Age, y | 53.46±10.33 | 54.59±9.29 | .462 |
| Age at study, years), mean±SD, | 65.58±10.56 | 66.92±9.49 | .397 |
| Hypertension | 32 (55.2) | 55 (45.5) | .223 |
| Diabetes | 10 (17.2) | 15 (12.4) | .382 |
| Dyslipidemia | 34 (58.6) | 56 (45.9) | .111 |
| Smoker | 29 (50) | 68 (56.2) | .436 |
| Family history of CAD | 13 (22.8) | 18 (15) | .202 |
| Presence of autoimmune disease | 7(12.1) | 10 (8.2) | .406 |
| Weight, kg | 83.96±12.68 | 83.09±13 | .670 |
| Height, cm | 170.3±7.32 | 169.98±7.66 | .790 |
| Body mass index, kg/m2 | 28.97±3.82 | 28.7±3 .96 | .661 |
| Clinical presentation | .060 | ||
| Stable | 22(37.9) | 28 (23) | |
| NSTEMI | 15(25.9) | 30 (24.6) | |
| STEMI | 21(36.2) | 64 (52.5) | |
| LVEF, % | 53.7±9.3 | 53.32±9.19 | .802 |
| SYNTAX score | 10.11±7.03 | 11.23±6.61 | .300 |
| No. of diseased vessels | 1.71±0.79 | 1.61±0.71 | .430 |
| No. of lesions | 2.4±1.68 | 2.3±1.5 | .699 |
| No. of stents | 1.78±1.22 | 2.02±1.12 | .195 |
CAD, coronary artery disease; LSS, long standing stable; LVEF, left ventricular ejection fraction; NSTEMI, non–ST-elevation acute myocardial infarction; RCP, rapid clinical progressor; SD, standard deviation; STEMI, ST-segment elevation myocardial infarction.
The data are presented as No. (%) or mean±standard deviation.
Serum levels of markers of inflammation/atherosclerosis and laboratory analytes in patients during a stable disease phase
| RCP(n=58) | LSS(n=122) | P | |
|---|---|---|---|
| Glucose, mg/dL | 115.9±36.45 | 112.69±27.79 | .515 |
| Creatinine, mg/dL | 1.06±0.35 | 0.92±0.4 | .030 |
| Triglycerides, mg/dL | 127.5 [77.25-192] | 115 [86-165.25] | .876 |
| Total cholesterol, mg/dL | 139.5 [118.25-160] | 150 [133.75-171.25] | .069 |
| LDLc, mg/dL | 76 [54.5-93.5] | 81.5 [68-97] | .094 |
| HDLc, mg/dL | 39 [36-43.75] | 42.5 [38-52.25] | .026 |
| Triglycerides/HDLc | 3.34 [1.80-4.90] | 2.82 [1.76-4.71] | .418 |
| Apo-A1, mg/dL | 130 [118-143.75] | 144.5 [129-161.25] | .001 |
| Apo-B 100, mg/dL | 68.5 [59.5-86] | 74.5 [65-87] | .060 |
| Phospholipids, mg/dL | 168.04±36.46 | 178.42±30.86 | .060 |
| Homocysteine, umol/L | 14.5 [11.92-20.77] | 14.7 [11.67-18.52] | .959 |
| LPa, mg/dL | 26 [11.8-85.27] | 26 [9.4-63] | .453 |
| PCSK9, ng/mL | 475.4 [409.3-606.8] | 459.3 [395.3-545] | .010 |
| LDL-R, pg/mL | 40 640 [28 585-49 480] | 41 130 [27 515-55 620] | .600 |
| SREBP2, ng/L | 155.5 [135.8-181.3] | 154.4 [128-182.7] | .580 |
| CRP, mg/dL | 0.27 [0.09-0.8] | 0.59 [0.1-1.82] | .078 |
| IL-6, pg/mL | 3.94 [2.45-7.22] | 3.08 [2.09-5.11] | .047 |
| TNF | NA | NA |
Apo, apolipoprotein; CRP, C reactive protein; HDLc, high-density lipoprotein; IL-6, interleukin-6; IQR, interquartile range; LDLc, low-density lipoprotein; LDL-R, low-density lipoprotein receptor; LPa, lipoprotein a; LSS, long standing stable; NA, not available; PCSK9, proprotein convertase subtilisin/kexin type 9; RCP, rapid clinical progressor; SREBP2, sterol regulatory element binding transcription factor 2; TNF, tumor necrosis factor.
Values are expressed as mean±standard deviation or median [interquartile range].
P values<.05 are highlighted in bold font.
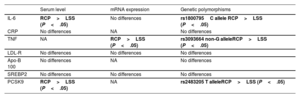
As previously explained, 7 molecules were analyzed at 3 different levels (protein levels, mRNA expression, and genetic polymorphisms) to allow a more complete approach to the disease. Table 3 displays at a glance the performance of each marker. Some showed no significant differences at any of the levels (LDL-R, Apo-B, SREBP2 and CRP). However, interleukin-6 and TNF in the inflammation flank and PCSK9 on the lipid side showed consistent and significant findings at more than one level. Serum TNF levels were very low and not properly detected. Interleukin-6 and PCSK9 levels were significantly higher in the RCP group (figure 1A,C), while apo-B100 and CRP tended to be lower in this group.
Findings of the molecules analyzed at the molecular 3 levels when comparing RCP vs LSS patients
| Serum level | mRNA expression | Genetic polymorphisms | |
|---|---|---|---|
| IL-6 | RCP>LSS (P<.05) | No differences | rs1800795C allele RCP>LSS (P<.05) |
| CRP | No differences | NA | No differences |
| TNF | NA | RCP>LSS (P<.05) | rs3093664 non-G alleleRCP>LSS (P<.05) |
| LDL-R | No differences | No differences | No differences |
| Apo-B 100 | No differences | NA | No differences |
| SREBP2 | No differences | No differences | No differences |
| PCSK9 | RCP>LSS (P<.05) | NA | rs2483205 T alleleRCP>LSS (P<.05) |
Apo, apolipoprotein; CRP, C-reactive protein; IL-6, interleukin-6; LDL-R, low-density lipoprotein receptor receptor; LSS, long standing stable; NA, not available; PCSK9, proprotein convertase subtilisin/kexin type 9; RCP, rapid clinical progressor; SREBP2, sterol regulatory element binding transcription factor 2; TNF, tumor necrosis factor.
P<.05 are highlighted in bold font.
Interleukin-6 (IL-6), tumor necrosis factor (TNF) and proprotein convertase subtilisin/kexin type 9 (PCSK9) findings at different molecular levels. The figure shows a comparison between the the rapid clinical progressor group and the long standing stable group at different molecular levels. IL-6 serum levels (A), TNF mRNA expression (B) and PCSK9 serum levels (C) are significantly higher in the rapid clinical progressor group. The horizontal line within the plots represents the mean. P values were adjusted by age, sex, diabetes mellitus, smoking, and hypertension. mRNA, messenger ribonucleic acid.
Apart from the higher serum interleukin-6 levels in the RCP group (P=.002) (figure 1A), differences were found in the genotypic and allelic frequencies of interleukin-6 rs1800795. In particular, patients with the CC genotype and C allele had a higher risk of RCP (OR, 2.70 [1.02-7.18]; P=.046; OR,1.71 [1.06-2.75], P=.028, respectively). Interestingly, the same finding was observed in patients with the GCG haplotype (OR, 2.75 [1.19-6.37]; P=.02) (table 2 of the supplementary data).
Expression of TNF mRNA was increased in the RCP group compared with the LSS group (P=.003; figure 1B). As for the TNF polymorphisms analyzed, rs3093664 showed differences in allelic and carrier frequencies. In both cases, the presence of the G allele was related to a lower risk of RCP (OR, 0.27 [0.08-0.92]; P=.04; OR, 0.27 [0.07-0.95]; P=.04) (table 3 of the supplementary date).
Among the 6 PCSK9 SNPs studied, rs2483205 showed significant differences in the genotypic and allelic frequencies (CT genotype: OR, 3.39 [1.50-7.65]; P=.003; TT genotype: OR, 3.12 [1.12-8.64]; P=.03; and T allele: OR, 1.83 [1.15-2.93]; P=.01), demonstrating that the presence of the T allele was associated with a higher risk of RCP (table 4 of the supplementary data).
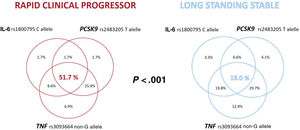
Figure 2 shows the prevalence of interleukin-6 rs180075C, TNF rs3093664 non-G and PCSK9 rs2483205 T alleles (isolated or combined) in the 2 groups. A total of 51.7% of patients with RCP had all 3 risk alleles compared with 18.0% of LSS patients (P<.001).
Distribution of the IL-6 rs180075C, TNF rs3093664 non-G and PCSK9 rs2483205 T alleles in RCP and long standing stable patients. Patients with the 3 risk alleles were more frequent in the RCP group (P<.001). IL-6, interleukin-6; PCSK9, proprotein convertase subtilisin/kexin type 9; TNF, tumor necrosis factor; RCP, rapid clinical progressor.
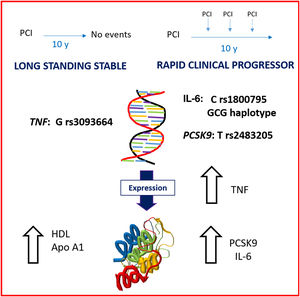
In summary, as reflected in the figure 3, at a clinically stable phase of the disease, patients with RCP had an underlying higher level of inflammation (both in interleukin-6 and TNF molecules) and higher serum levels of PCSK9, while LSS patients had a more protective lipid profile (higher levels of high-density lipoprotein and apo-A1). The combination of all the 3 risk alleles was more prevalent in the RCP group.
Central illustration. Global vision of the case and control groups showing the main differences. In a stable phase of the disease, rapid clinical progressors show an underlying higher level of inflammation (both in interleukin-6 and tumor necrosis factor molecules) and higher serum levels of proprotein convertase subtilisin/kexin type 9, whereas long standing stable patients have a more protective lipid profile (higher levels of high-density lipoprotein and apolipoprotein-A1). Several polymorphisms were also identified as markers of higher or lower risk of clinical progression.
Our study shows several interesting findings: a) the profiles of both groups of patients at first cardiovascular event were very similar except for clinical presentation, as patients with RCP more frequently presented with stable angina than LSS patients whose first clinical event was more often ST-elevation myocardial infarction. This difference was not statistically significant, but that could be the result of a type II error. The remaining variables analyzed were remarkably similar between groups. b) The lipid profile also showed interesting results. Apo-B100 and low-density lipoprotein cholesterol levels tended to be lower in the RCP group, most likely related to a more enhanced statin therapy in this group. In contrast, high-density lipoprotein and Apo-A1 levels were clearly lower in the RCP group, which could be explained by a higher-risk underlying condition in the RCP population. c) Serum PCSK9 levels were significantly higher in the RCP group. This, along with the genetic findings (higher presence of T allele of the rs2483205 polymorphism in this group) aligns with the idea of an underlying higher-risk condition in these patients. d) Our findings on the key role played by inflammation in coronary atherosclerosis were consistent, even at a stable disease phase. Both interleukin-6 and TNF levels were significantly increased in RCP patients. This finding was mirrored at the genetic level. Significant differences were found in the frequencies of the alleles of the TNF rs3093664 polymorphism and the interleukin-6 rs1800795 polymorphism. e) There is a likely synergy between different pathways, as the combination of the 3 risk alleles is the pathway specifically related to RCP.
Patients with clinically evident coronary atherosclerosis have a highly variable rate of progression and progression is one of the most important factors influencing prognosis. This variability partially depends on traditional risk factors, but to enhance understanding, there is a need to investigate the -omics, the molecular phenotype, and the genotype. This aligns with the emerging approach to disease treatment and prevention of precision medicine, which takes into account individual variability in genes, the environment, and lifestyle. In this study, we intended to follow this path, going beyond traditional risk factors to focus on the -omics with a view to identifying differences in patients’ substratum and heading toward “precision prevention” where the intensity and type of interventions can be tailored to specific individuals.
Inflammatory cells and lipoproteins interact with biochemical products in the vascular wall and lead to the formation of atheroma plaque. Subsequent changes in the composition of the atherosclerotic plaque and in inflammation define the course to stable or unstable events.
Interleukin-6 and CRP are two of the molecules involved in the most widely tested inflammatory condition. Several studies suggest that these two molecules are associated with the long-term risk of cardiovascular events in patients with established coronary artery disease or patients at risk of atherosclerosis.6,31–36
Data from clinical trials and meta-analyses suggest that there is a consistent relationship between low levels of low-density lipoprotein (LDL) cholesterol and a lower rate of major cardiovascular events.24,25 Recycling of LDL receptors (LDL-R) on hepatocyte surfaces plays a crucial role in maintaining the balance of body and cellular cholesterol, thus regulating plasma levels of LDL cholesterol. It has been recently shown that PCSK9 is essential in the regulation and recycling of LDL-R. Monoclonal antibodies that inhibit PCSK9 have emerged as a new class of drugs that effectively lower LDL cholesterol levels,19 have a favorable effect on progression of coronary atherosclerosis,20 and positively impact the rate of cardiovascular events.21–23 Of note, PCSK9 expression increases the production of inflammatory mediators, such as TNF, NF-κB, and interleukin-1, among others.24 SREBP2 acts in cholesterol homeostasis, regulating both the expression and synthesis of LDL-R and PCSK925–29 and has also been involved in the atherogenesis process.
Genetic studies have recently identified SNP as a natural form of genetic diversity among humans and, with advances in genotyping, several genetic polymorphisms have been identified in relation to a phenotypic effect. Many SNPs that are located in promoter regions have a functional impact through significant changes in levels of gene product expression. Accordingly, several studies have related SNPs of the interleukin-6 and CRP genes with clinical features of cardiovascular disease.37–45 SNPs on the PCSK9 gene have also been tested in several studies proving a clear relationship between disorders in the secretion and/or function of this gene with plasma LDL levels and therefore cardiovascular events.44,45 However, although we analyzed CRP and lipoprotein(a), we found no difference between groups, unlike other authors.5,7 Konstantinos et al.,32 focused their study on the C allele of interleukin-6 rs1800795 polymorphism and they found an association with angiographic progression of coronary artery disease. We detected the same association in our study but, in our case, with clinical progression.
Other authors are also in search of novel targets in the field of lipids beyond traditional risk factors that could impact rapid progression of the cardiovascular disease, always from an anatomical perspective. Sekimoto et al.,46 have recently published a highly interesting article analyzing the role of small dense LDL in the risk of rapid angiographic progression of nonculprit coronary lesions after an acute coronary syndrome. Like us, they found no differences in the baseline characteristics and traditional risk factors. However, levels of high-density lipoprotein, apo-A1 and apo-B100 were higher in their rapid progression group both right after the acute coronary syndrome and during a stable phase, unlike our findings. These authors concluded that small dense LDL levels are associated with rapid progression in the setting studied. Won et al.,12 focused their study on the role of the atherogenic index of plasma, concluding that it is an independent marker of rapid plaque progression.
It would be of great interest to continue this line of research with a prospective validation cohort in wider populations in order to assess whether this molecular phenotype and genotype profile could predict more aggressive disease presentations and eventually address them with an earlier and more intense treatment strategy. This, among other actions, could involve achieving optimal LDL levels earlier, increasing the number of periodic follow-up visits, and even performing follow-up with imaging techniques, as well as encouraging patient awareness of the disease.
LimitationsThe sample size of this study is not large, although it is fairly similar to those of other studies addressing rapid angiographic progression and its relationship with various biologic markers. The patients were studied in a stable stage of the coronary artery disease instead of during an acute event, in which we might have found more differences in markers of inflammation. However, the decision to study patients in a stable stage was deliberate as we sought to identify some underlying baseline condition that migh define patients over the life course. Thus, we decided to avoid the acute stage of the coronary disease. Because this study was retrospective, we missed all the nonsurvivors who might have been included in the RCP group and who might have had a more aggressive disease presentation. We acknowledge that this study did not capture all cases of RCP. On the other hand, all the patients in the group were truly RCP. Finally, RCP was defined for the purposes of this study. We found no proper definition of rapid progression from a clinical perspective in the literature, and therefore decided to choose one, based on our perception as clinicians. This definition could, nonetheless, have been different.
The prevalence of the 3 risk alleles identified in the RCP group suggests a synergy between several activated pathways. Nonetheless, although there seems to be a certain degree of genetic conditioning, this same prevalence leaves room for other nongenetic determinants particular to atherosclerosis per se.
All these findings will need validation in further prospective cohort studies.
CONCLUSIONTo the best of our knowledge, our study is the first to address the rapid clinical progression of coronary atherosclerosis. The results of this study suggest the existence of specific baseline phenotypic and genotypic markers associated with rapid progression of coronary artery disease. Further prospective cohort studies are warranted to determine whether these profiles could be used to implement a precision secondary prevention strategy in patients with coronary artery disease.
- •
The rate of coronary atherosclerosis progression impacts prognosis.
- •
Accelerated coronary atherosclerosis might be better defined from a clinical perspective.
- •
Patients with proven stability share classic features with those with aggressive presentations.
- •
The interleukin-6 rs180075C, TNF rs3093664 non-G and PCSK9 rs2483205 T alleles are associated with rapid clinical progression.
This study was supported by an unrestricted grant from AMGEN. S. Remuzgo-Martínez is supported by funds of RETICS Program (RD16/0012/0009) from the Instituto de Salud Carlos III (ISCIII), cofunded by the European Regional Development Fund (ERDF). F. Genre is supported by funds from the RICORS Program (RD21/0002/0025) from ISCIII, cofunded by the European Union. R. López-Mejías is a recipient of a Miguel Servet type II Program fellowship from the ISCIII, cofunded by the European Social Fund, “Investing in your future” (CPII21/00004). V. Pulito-Cueto is supported by funds from PI18/00042 from ISCIII, cofunded by ERDF.
AUTHORS’ CONTRIBUTIONST. García-Camarero, M.Á. González-Gay and J.M. de la Torre Hernández designed the study; T. García-Camarero, G. Veiga Fernández, D.-H. Lee Hwang, F. Sainz Laso and A. Gil Ongay recruited the patients; T. García-Camarero and J.M. de la Torre Hernández conducted the research; S. Remuzgo-Martínez, F. Genre, R. López-Mejías and V. Pulito-Cueto conducted the laboratory analysis: T. García-Camarero, S. Remuzgo-Martínez, F. Genre, R. López-Mejías and V. Pulito-Cueto performed the statistical analysis; T. García-Camarero and J.M. de la Torre Hernández drafted the manuscript; T. García-Camarero has primary responsibility for final content. J.M. de la Torre Hernández, S. Remuzgo-Martínez, F. Genre, R. López-Mejías and V. Pulito-Cueto contributed to the data interpretation and commented on the initial versions of the manuscript. All authors read and approved the final manuscript.
CONFLICTS OF INTERESTThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2023.04.005