Keywords
INTRODUCTION
Heart failure is a complex syndrome that causes progressive widespread organ damage.1 Although the pathophysiologic mechanisms are not well understood,2 peripheral muscle wasting is commonly associated with chronic advanced heart failure and its presence is independently associated with worse prognosis.3
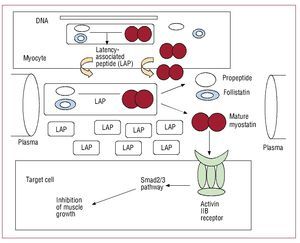
Myostatin is a protein inhibitor of muscle growth belonging to the transforming growth factor b family of growth factors. It is expressed in muscle tissue and plays a fundamental role in the regulation of muscle mass (Figure 1). Although myostatin has been reported to be elevated in certain muscle wasting conditions,4-6 no data are available on its role in patients with chronic heart failure.
Figure 1. Synthesis, release, and mechanism of action of myostatin and its propeptide precursor.
The aim of this study was to analyze the serum concentration of myostatin and its propeptide precursor (promyostatin) in patients with chronic heart failure and to analyze its relationship with functional class, various clinical and laboratory variables, and mortality.
METHODS
Seventy outpatients with chronic heart failure and systolic dysfunction were enrolled according to functional class: 30 patients with New York Heart Association functional class I-II and 40 with functional class III-IV. A routine appointment was made and venous blood samples were taken. Samples were centrifuged for 20 minutes at 3000 rpm and the serum was frozen at -80°C. Patient follow-up appointments were programmed according to the protocol used in the unit. Serum concentration of promyostatin was determined by enzyme-linked immunosorbent assay (ELISA) using the myostatin LAP/propeptide kit (BioVendor, Czech Republic). The detection limit of the kit is 0.023 ng/mL and the linear range of detection, 0.1-10 ng/mL. As measures of precision, the within-test coefficient of variation (CV) is between 2.9% and 5.4%, and the between-test CV is 8.6% to 10.6%. The serum concentration of myostatin was determined using the Human Myostatin ELISA kit (Immundiagnostik AG, Bensheim, Germany). The detection limit of the kit is 0.273 ng/mL and the linear range 0 to 250 ng/mL (between-test CV, 3%). In the absence of a reference range for the concentration of these proteins, a control group was established comprising healthy volunteers (7 men and 4 women; mean [SD] age, 66.9 [9.8] years) with no history of heart disease, myopathy, or hormonal abnormalities. There were no differences between patients and controls in terms of age or sex. Serum concentrations of r2-TNFa and NT-proBNP were determined using the TNF-R (60 kDa) Instant ELISA (Bender Med Systems GmBH, Austria) and Dimension RxL (Dade Behring) analyzer, respectively.
Statistical analysis was performed using SPSS 11.0 for Windows. The concentration of myostatin was normally distributed, but those of promyostatin, r2-TNFa, and NT-proBNP were not. Continuous variables were expressed as means (SD) or medians (interquartile range), according to whether or not they were normally distributed. The relationship between these continuos variables and clinical and laboratory parameters, functional class, and mortality were measured using the appropriate statistical test (t test, Mann-Whitney U test, and Pearson or Spearman correlation coefficients). Multivariate analysis was performed by Cox regression survival analysis ("enter" method) and included the following covariables: age, sex, functional class, ejection fraction, and concentrations of myostatin, promyostatin, r2-TNFa, and NT-proBNP. The cutoff for statistical significance was set at P<.05. No patients were lost to follow-up and, at the end of the study period, vital status was determined for all patients.
The study complied with international recommendations on clinical research according to the Declaration of Helsinki. All patients provided signed informed consent to participate in the study, which was approved by the institutional review board.
RESULTS
Patient characteristics are shown in the Table. The mean concentration of myostatin was 12.3 (7) ng/ mL and the median concentration of promyostatin was 0.1 (0.1-0.38) ng/mL. The median concentration of r2-TNFa was 2.42 (1.61-4.13) ng/mL and that of NT-proBNP, 899 (290-2743) pg/mL. Comparison of the concentrations measured in patients with those from healthy volunteers revealed that patients had lower concentrations of both myostatin (12.3 [7] vs 18.6 [9.3] ng/mL; P=.01) and promyostatin (0.1 [0.1-0.38] vs 1.25 [0.1-2.9] ng/mL; P=.02).
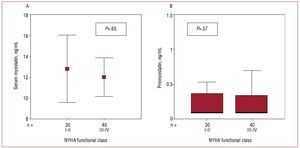
There was no relationship between NYHA functional class and the concentration of myostatin (Figure 2A) or promyostatin (Figure 2B). In contrast, functional class was correlated with both r2-TNFa (P=.001) and NT-proBNP (P<.001) concentration. There was no correlation between myostatin and promyostatin concentration (r=-0.049; P=.68). Neither were correlated with r2-TNFa concentration (myostatin, P=.86; promyostatin, P=.65), NT-proBNP (myostatin, P=.75; promyostatin, P=.89), or with age, sex, ejection fraction, etiology of heart failure, duration of heart failure, glucose, total protein, albumin, creatinine, troponin I, urates, cholesterol, or body mass index, or with any of the treatments received. There was a weak correlation between the serum concentration of creatine kinase and that of promyostatin (r=0.24, P=.04) but not myostatin.
Figure 2. Relationship between New York Heart Association (NYHA) functional class and serum concentrations of myostatin (A) and promyostatin (B).
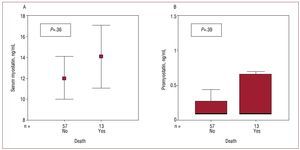
Thirteen patients died during follow-up (17.9 [1.3] months): 9 due to heart failure, 2 due to sudden death, 1 due to other cardiovascular causes, and 1 due to noncardiovascular causes. There was no relationship between either myostatin or promyostatin concentration and death (Figure 3). However, patients who died had higher concentrations of NT-proBNP (2998 [2610-9983] vs 573 [238-1582] pg/ mL; P<.001) and r2-TNFa (4.32 [1.94-7.51] vs 2.30 [1.43-3.27] ng/mL; P=.005). In the Cox regression analysis, functional class (hazard ratio [HR], 5.28 [95% confidence interval, 1.47-19.0]; P=.01) and concentration of r2-TNFa (HR, 1.24 [1.03-1.49]; P=.02) were associated with mortality.
Figure 3. Relationship between all-cause mortality and serum concentrations of myostatin (A) or promyostatin (B).
DISCUSSION
Myostatin has been linked to muscle wasting in a variety of contexts.4-6 It has been suggested that this protein could be implicated in the organ damage associated with heart failure.7 However, in our study, we found lower concentrations of myostatin in patients with heart failure than in healthy volunteers. This completely unexpected finding may suggest that myostatin expression is downregulated in an attempt to conserve muscle mass, although this is merely speculation.
Our study also showed no relationship between the concentration of myostatin or promyostatin and functional class or other parameters that are commonly used to assess the severity of heart failure; furthermore, there was no relationship between either of these measures and mortality. In contrast, the concentrations of both r2-TNFa and NT-proBNP were clearly associated with functional class and survival. The absence of an association between myostatin or promyostatin and these variables could indicate that, although these proteins are implicated in muscle damage associated with heart failure, their concentration is independent of disease severity, or even that they are not involved in this organ damage. Very little is known about the pathophysiology of this protein and very little information has been reported on its serum concentration. Analysis of the results obtained in different studies is complicated by the use of antibodies against different regions of the protein and the fact that 70% of circulating myostatin is bound to its propeptide.8 There is also a lack of agreement regarding the relationship between serum concentrations and concentrations in skeletal muscle, although some studies have reported them to be similar.4,5 It has been reported previously that the serum concentrations of the propeptide are very low,9 a finding that was confirmed in our study. Furthermore, beta blockers have been reported to have an anabolic effect.10 Consequently, the absence of a relationship between myostatin concentrations and severity of heart failure may have been conditioned by the large proportion of patients receiving beta blockers.
Limitations
The number of patients analyzed and the number of events were very low. The group of healthy volunteers was small and was not controlled for population variables. Objective data for the confirmation of functional class, such as stress testing with gas exchange or the 6-minute walk test, were unavailable.
In conclusion, the concentrations of both myostatin and promyostatin measured by ELISA showed no relationship with markers of severity or prognosis in patients with chronic heart failure.
ACKNOWLEDGMENTS
The authors are grateful to Josep Maria Manresa for help with statistical analysis.
This study was supported by an unrestricted grant from Laboratorios Menarini S.A., Spain.
Correspondence: Dr. E. Zamora.
Unidad de Insuficiencia Cardiaca. Servicio de Cardiología. Hospital Universitario Germans Trias i Pujol.
Ctra. del Canyet, s/n. 08016 Badalona. España.
E-mail: e.zamora@telefonica.net
Manuscript received March 28, 2009.
Accepted for publication September 2, 2009.