The rapidly progressive aging of Western countries suggests that about one-third of the population will be older than 65 years in 2050.1 Thus, one of the biggest challenges in modern cardiology involves determining the risk of atrial fibrillation (AF) and identifying which patients should receive anticoagulation therapy.2,3 Treatment of AF with anticoagulants, performed since the late 1980s,4 has significantly reduced the incidence of stroke. However, their exact impact on cognitive impairment is still unknown, although recent evidence has been published on the relationship between AF and cognitive decline.5 In addition, there are new data6,7 on the identification of at-risk patients and the importance of AF as a causal factor for embolisms. The information provided by continuous cardiac monitoring devices (implantable loop recorders, pacemakers, and defibrillators) has indicated that there is no temporal relationship between AF and stroke. In fact, almost no patients with paroxysmal AF have AF at the time of stroke onset, although AF is indeed a marker of endothelial dysfunction and another risk factor for stroke.8–11 Recent work has also strengthened the value of surface ECG, specifically ECG of interatrial block (IAB) disorders, which can help to determine the risk of AF.
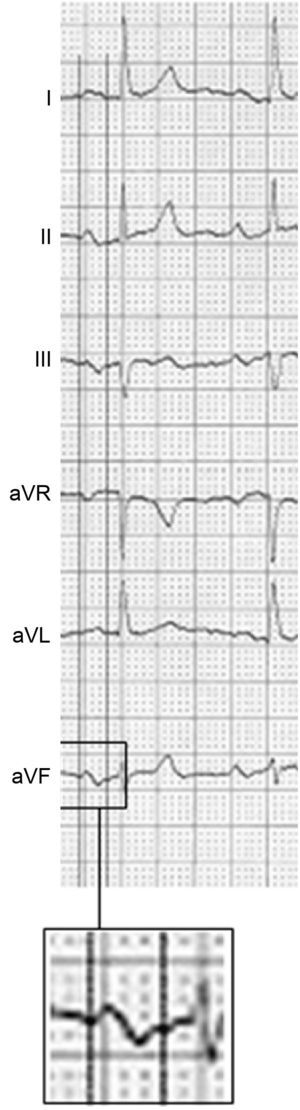
The diagnosis and classification of IABs (partial and advanced) was published in the 1980s by Bayés de Luna12,13 and later by the group of Spodick,14 among others. Their diagnostic criteria were finally defined in a consensus document in 2012.15 Partial IAB is diagnosed in the presence of a P wave ≥ 120ms, whereas the more advanced form is diagnosed if there is also a ± pattern in leads II, III, and aVF (Figure 1). In addition, ± P from V1 to V3 is almost always present, but this criterion can also be found in isolated left atrial enlargement. When advanced IAB shows a very long P wave (≥ 160 ms) and the patient has obvious structural heart disease or elevated CHA2DS2-VASc11 (heart failure or systolic dysfunction, hypertension, age ≥ 75 years, diabetes mellitus, stroke, vascular disease, age 65-74 years, female sex) and ambient atrial arrhythmias (more than 40 atrial premature beats/h on Holter monitoring), there is a very high possibility of AD/atrial flutter development in the next 2 to 3 years. Various studies have confirmed the usefulness of advanced IAB as a predictor of AF.16–22 Recently, this condition was named Bayés syndrome.23–26 Finally, IABs and ambient arrhythmias are also frequently associated with stroke and cognitive impairment.26–31
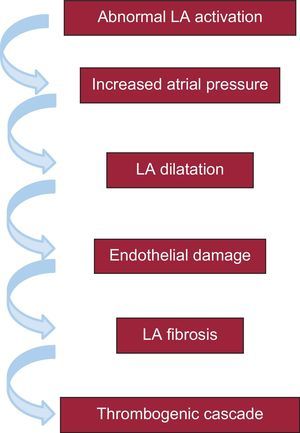
The pathophysiology of the association between advanced IAB and AF and stroke probably depends on a series of sequential electromechanical changes that, starting with an abnormal and delayed activation of the left atrium, culminates in a thrombogenic cascade, primarily in the left atrial appendage32 (Figure 2). It is interesting to note the important role played by fibrosis in this mechanism, because fibrosis has already been closely related to endothelial damage and promotes the development of AF and thrombogenic cascades.33–38
This sequence of events has led to the conclusion that it would be pertinent to identify patients with advanced IAB who have the characteristics included in the Table, because these patients have high risk of AF and stroke. They would thus benefit from the early use of anticoagulation therapy, even without evidence of AF. This approach has been strengthened6,7 by the recent demonstration that, although patients with a high CHA2DS2-VASc score show an increased prevalence of AF, thromboembolic complications appear to be independent of the presence of AF when this score is very high. If advanced IAB is also present, it would give rise to a subgroup of patients who will probably show an even higher risk of short-term embolic complications with or without the development of AF. This strategy might at least partly avoid the cognitive impairment that appears to be associated with AF, but that surely should be considered linked to the microembolic processes frequently occurring in these patients before a stroke.5
The hypothesis of anticoagulating patients in sinus rhythm showing the characteristics of the Table but without confirmed AF seems to us to be obvious and appealing. In addition, the clinical application of this approach could already be considered in certain patients. Moreover, the diagnosis of IAB is simple and only requires electrocardiographic testing.39 However, 2 prerequisites must be met before this approach can be generally recommended. The first involves the creation of a registry including patients with advanced IAB and the characteristics listed in the Table and a control group comprising patients with a similar clinical profile but with partial IAB. The second is that, if this registry shows that patients with advanced IAB have a higher risk of stroke regardless of whether they have documented AF or not, a clinical trial should be performed with anticoagulants (possibly using the “new” oral anticoagulants) and a control group to determine if anticoagulation therapy can reduce the incidence of stroke and cognitive decline in these patients. If so, anticoagulant therapy would be advisable for these patients because, although it would not avoid the development of AF or atrial flutter, it could reduce the collateral effects and sequelae (stroke and cognitive impairment). As Fabritz has recently noted, a small biphasic P wave in the elderly could have a considerable impact.40
CONFLICTS OF INTERESTNone declared.