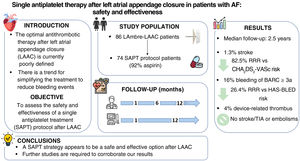
The optimal antithrombotic strategy following left atrial appendage closure (LAAC) is poorly defined in patients with nonvalvular atrial fibrillation. We assessed the safety and effectiveness of a single antiplatelet treatment (SAPT) strategy after LAAC in a population at high risk of ischemic and bleeding events.
MethodsThis single-center, observational, prospective study included a consecutive cohort of patients who underwent LAAC using the LAmbre device (Lifetech Scientific, China) and who were discharged with SAPT. The primary outcome was a composite of stroke, systemic embolism, and device-related thrombosis during follow-up. Secondary endpoints were cardiovascular mortality and major bleeding events (BARC ≥3a). Clinical follow-up was performed at 1, 6, and 12 months and subsequently on an annual basis. Transesophageal echocardiography was performed at 1 and 12 months of follow-up.
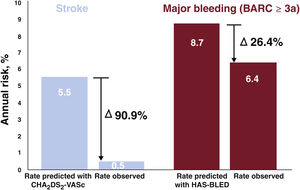
ResultsThe study comprised 74 patients. The median age was 77 [72-83] years and 43% were women. The cohort exhibited a high prevalence of comorbidities and cardiovascular risk factors. The median CHA2DS2-VASc and HAS-BLED scores were 4 [3-6] and 4 [4-5], respectively. The median length of follow-up was 2.5 years (188 patients-year). During follow-up, device-related thrombosis occurred in 3 patients (4%). Ischemic stroke occurred in 1 patient (1.3%, rate 0.5%/y), representing a 90.9% relative risk reduction compared with the risk predicted by CHA2DS2-VASc. Major bleeding events occurred in 12 patients (16%, 6.4%/y), with a relative risk reduction of 26.4% of that predicted by HAS-BLED. Cardiovascular-related mortality was observed in 2 patients (2.7%).
ConclusionsSAPT appears to be a safe and effective treatment following LAAC in patients at high ischemic and hemorrhagic risk. Further studies are needed to confirm our findings.
Keywords
In recent years, left atrial appendage closure (LAAC) has become an effective therapeutic option for the prevention of thromboembolic events in patients with nonvalvular atrial fibrillation (AF) and contraindication to oral anticoagulants (OACs) or with very high bleeding risk (IIb B indication).1 Since 2001, multiple devices have been developed and found to both progressively improve procedural outcomes and prevent embolic events in the long term.2–5 The optimal antithrombotic regimen for avoiding device-related thrombosis (DRT) after LAAC is currently unknown. The presence of this complication is associated with an increased incidence of stroke during the follow-up of these patients.6,7 In theory, the antithrombotic treatment should be sufficiently potent to avoid DRT while being as simple and short as possible to minimize bleeding complications in this group of patients with high bleeding risk.
The proposed post-LAAC regimens have differed over time and according to the type of device used. The strategies have evolved from more aggressive initial regimens with the WATCHMAN device (Boston Scientific, United States) in patients without previous contraindication to OACs (6 weeks’ treatment with OACs+aspirin, followed by dual antiplatelet therapy [DAPT] for 6 months and then indefinite aspirin)5 to the current simpler regimens for patients with a contraindication to OACs, which are generally recommended to comprise a minimum of 3 months of DAPT followed by indefinite single antiplatelet therapy (SAPT).8–11 Despite the recommendations, some real-life clinical practice studies have found the need to simplify and shorten these treatments even further because, on the one hand, the patients undergoing LAAC have higher bleeding risk than those included in the fundamental studies of these devices12–14 and, on the other, simpler antithrombotic regimens do not seem to be related to more thromboembolic events during follow-up.15 Thus, the objective of the present study was to assess the safety and effectiveness of a SAPT regimen after LAAC conducted with a latest-generation device in the prevention of thromboembolic and bleeding events during follow-up.
METHODSStudy designThe present single-center, observational, prospective, and nonrandomized study included a consecutive cohort of patients who underwent LAAC using the LAmbre device (Lifetech Scientific Co, China) and were discharged with SAPT.
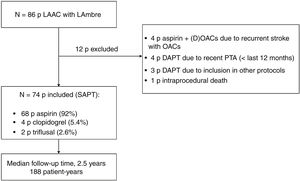
Patient selection and proceduresThe inclusion criteria were age ≥ 18 years, diagnosis of nonvalvular AF with indication for indefinite OACs, and indication for LAAC due to absolute or relative contraindication to adequate doses of prolonged anticoagulants (approved by an expert committee based on patient history and current clinical practice guidelines). All consecutive patients between May 2017 and March 2021 were enrolled. The exclusion criteria were suboptimal device implantation and need for DAPT or OACs for other medical reasons (such as percutaneous coronary or vascular revascularization in the last 12 months). All patients underwent LAAC with the LAmbre device during the study recruitment period. During this period, no other devices were used for LAAC. Figure 1 describes the population included in the study.
The procedures were performed by interventional cardiologists who were experts in LAAC with the support of an anesthesia and cardiac imaging team. Transesophageal echocardiography (TEE) or intracardiac echocardiography (8%) was conducted in all patients to rule out the presence of left atrial appendage thrombosis and guide the procedure. At 24hours after the procedure, transthoracic echocardiography was performed to ensure correct device placement and exclude pericardial effusion. If no complications occurred, patients were discharged at 24hours with SAPT (aspirin, clopidogrel, or triflusal).
Research ethicsThe study protocol was approved by the Ethics Committee for Medical Research of the institution (CEIm-PSMAR). All patients signed an informed consent form before the procedure. All study procedures were conducted in accordance with the principles of the Declaration of Helsinki and ISO 14155 guidelines.
Baseline assessmentWe collected baseline clinical data (sociodemographic data, medical history, and ischemic and bleeding risk according to the CHA2DS2-VASc16 and HAS-BLED17 scales), blood test data (renal function and complete blood counts), and echocardiographic data (measurement of the left atrial appendage and ventricular function and determination of valvular heart diseases). Technical data were collected during the LAAC (size of device used, procedural time, size of left atrial appendage on fluoroscopy, and procedural success), as well as clinical data (length of hospital stay and local and in-hospital complications).
Follow-up and event definitionIn-person clinical follow-up was conducted at 3 and 12 months after the LAAC. All patients were treated with SAPT for at least 1 month after the procedure. SAPT discontinuation was at the discretion of the treating cardiologist based on relevant medical history (eg, ischemic heart disease, peripheral vascular disease), the patient's bleeding risk (based on previous events and their severity, as well as HAS-BLED score), and TEE data during follow-up (presence of residual leaks).
Device monitoring was conducted using TEE imaging at 1 and 12 months after the LAAC. The presence of periprosthetic leaks and their size were recorded, as well as images indicating DRT. DRTs were confirmed using synchronized cardiac computed tomography (CT). If thrombosis was found, it was directly reported to the team for early assessment and treatment.
Primary endpoints were defined as a composite of ischemic events during follow-up: stroke, transient ischemic attack, systemic embolism, and DRT. Cardiovascular and all-cause mortality were also recorded.
Secondary endpoints were the presence of bleeding events during follow-up. Bleeding events were classified according to the mean Bleeding Research Academy Consortium (BARC) score,18 and major bleeding was defined as any event catalogued as BARC ≥3a (3a: overt bleeding with a hemoglobin drop >3g/dL or need for transfusion with overt bleeding; 3b, overt bleeding with a hemoglobin drop >5g/dL or need for surgery/vasopressors; 3c, intraocular or intracranial bleeding; 5, fatal bleeding).
Statistical analysisStatistical analysis was performed with IBM SPSS Statistics version 25. Continuous variables are expressed as mean ± standard deviation or median [interquartile range] according to distribution normality. Categorical variables are expressed as frequencies and percentages. Events during follow-up are expressed as the percentage of the total and as the event rate per 100 patient-years. The predicted rate of ischemic stroke was calculated from the CHA2DS2-VASc score adjusted for aspirin.19 The predicted rate of major bleeding was calculated according to the overall cohort of the HAS-BLED risk score.17 The relative risk reduction was calculated as (estimated annual rate %−observed annual rate %) / estimated annual rate %.
RESULTSStudy populationOverall, 86 patients underwent LAAC with the LAmbre device between May 2017 and March 2021; of these, 74 were enrolled in the study. Thus, 12 patients were excluded; the reasons for their exclusion are summarized in figure 1.
Baseline characteristicsThe baseline data and medical history of the patients included in the study are summarized in table 1. The population had a median age of 77 [72-83] years and a high prevalence of comorbidities (hypertension, 92%; type 2 diabetes mellitus, 49%; chronic kidney disease, 72%) and very high ischemic and bleeding risks (CHA2DS2-VASc, 4 [3-6]; HAS- BLED 4 [4-5]).
Baseline characteristics of the study participants (n=74)
| Variables | |
|---|---|
| Age, y | 77 [72-83] |
| Women | 32 (43.2) |
| Hypertension | 68 (92.0) |
| Diabetes | 36 (48.6) |
| AF type | |
| Paroxysmal | 34 (45.9) |
| Persistent | 5 (6.8) |
| Permanent | 35 (47.3) |
| CHA2DS2-VASc | 4 [3-6] |
| HAS-BLED | 4 [4-5] |
| Coronary heart disease | 22 (30.0) |
| AMI | 10 (13.5) |
| Peripheral vascular disease | 24 (32.4) |
| Stroke/TIA | 23 (31.0) |
| Previous major bleeding | 60 (81.0) |
| Gastrointestinal | 40 (66.7) |
| Hemorrhagic stroke | 12 (20.0) |
| Intracranial | 4 (6.7) |
| Abdominal/retroperitoneal | 1 (1.7) |
| Others | 3 (5.0) |
| Renal failure (GFR <60 mL/min/m2) | 53 (71.6) |
| Stage 3a (GFR <60 mL/min/m2) | 17 (32) |
| Stage 3b (GFR <45 mL/min/m2) | 12 (22.6) |
| Stage 4 (GFR <30 mL/min/m2) | 11 (20.7) |
| Stage 5 (GFR <15 mL/min/m2) | 13 (24.5) |
| Hemodialysis | 10 (13.5) |
| LA diameter | 45 [41-48] |
| Indication for LAAC | |
| History of major bleeding with OACs | 60 (81) |
| End-stage renal disease and high bleeding risk | 13 (17.6) |
| Severe liver disease | 1 (1.4) |
AF, atrial fibrillation; AMI, acute myocardial infarction; GFR, glomerular filtration rate; LA, left atrium; LAAC, left atrial appendage closure; OACs, oral anticoagulants; TIA, transient ischemic attack.
Data are expressed as No. (%) or median [interquartile range].
In total, 81% of patients had already had at least 1 major bleeding event; the most frequent origin was gastrointestinal (66.7%), followed by hemorrhagic stroke (20%). In addition, 31% of the patients had already experienced 1 cerebral bleeding event (stroke/transient ischemic attack). Three patients (4%) developed an acute complication during the procedure: 2 had femoral hematoma (not requiring surgical intervention) and 1 had cardiac tamponade requiring urgent pericardiocentesis that resolved. All patients were discharged after the procedure with SAPT (92% with aspirin).
Imaging follow-upTEE-mediated follow-up is summarized in table 2. TEE was obtained at 1 month of follow-up after the LAAC in 90.5% of patients. All patients had a technically optimal outcome after the closure, with a 0% rate of significant periprosthetic leaks (≥5mm). An image suggesting DRT was obtained from 1 patient (1.5%); this was confirmed with cardiac CT. This patient did not experience ischemic or embolic events during follow-up.
Imaging results at 1 month and 1 year (n=74)
| Variables | |
|---|---|
| TEE at 1 mo | 67 (90.5) |
| Device thrombosis* | 1 (1.5) |
| Peridevice leak | 14 (21.0) |
| Size of peridevice leak | 2.8±0.8 |
| <3 mm | 6 (9) |
| 3-4 mm | 8 (12) |
| ≥ 5 mm | 0 |
| Location of peridevice leak | |
| Marshall | 13 (93.0) |
| Mitral | 1 (7.0) |
| TEE at 12 mo | 52 (70.2) |
| Device thrombosis* | 2 (3.85) |
| Peridevice leak | 11 (21.1) |
| Size of peridevice leak | 2.45±0.82 |
| <3 mm | 6 (11.5) |
| 3-4 mm | 5 (9.6) |
| ≥ 5 mm | 0 (0) |
| Location of peridevice leak | |
| Marshall | 10 (91) |
| Mitral | 1 (9.0) |
TEE, transesophageal echocardiography.
Follow-up TEE was performed at 12 months in 70.2% of patients; none had significant periprosthetic leaks while 2 (3.85%) had images compatible with DRT. Neither of these patients experienced ischemic or embolic events before or after their diagnosis. The patients with DRT were treated with low-dose OACs (apixaban 2.5mg/12h) until the leak resolved on cardiac CT.
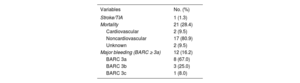
Clinical follow-upAll patients completed follow-up (table 3). The median follow-up duration was 2.5 [1.65-3.38] years, giving a total of 188 patient-years. SAPT duration was a median of 6 [3-30] months. One patient (1.3%) developed an ischemic stroke during follow-up, giving an annual incidence of 0.5%: this event occurred 2 years after the LAAC. Given the expected incidence of stroke according to CHA2DS2-VASc score in our embolic events during follow-up. cohort of 5.5%/y,19 this represents a relative risk reduction of 87.5% (figure 2).
Clinical events during follow-up (n=74)
| Variables | No. (%) |
|---|---|
| Stroke/TIA | 1 (1.3) |
| Mortality | 21 (28.4) |
| Cardiovascular | 2 (9.5) |
| Noncardiovascular | 17 (80.9) |
| Unknown | 2 (9.5) |
| Major bleeding (BARC ≥ 3a) | 12 (16.2) |
| BARC 3a | 8 (67.0) |
| BARC 3b | 3 (25.0) |
| BARC 3c | 1 (8.0) |
BARC, Bleeding Academic Research Consortium; TIA, transient ischemic attack.
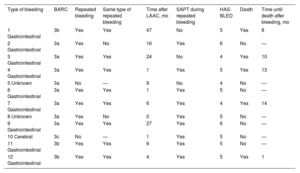
Regarding the secondary endpoints, 12 patients (16.2%; annual incidence, 6.4%) developed major bleeding during follow-up (BARC ≥ 3a). The theoretical incidence of bleeding according to the median HAS-BLED score was 8.7%/y, which represents a relative risk reduction of 26.4% vs the actual incidence of bleeding events (figure 2). There were no fatal bleeding events or bleeding requiring urgent surgical or endoscopic intervention. Notably, 10 of these 12 patients (83%) had a history of bleeding and 80% of these 10 patients had repeated bleeding of the same origin (gastrointestinal). In addition, bleeding events were associated with high mortality during follow-up (41.6%), with a mean of 9 months until death after the bleeding event. Bleeding events are described in greater detail in table 4. Two patients (2.7%) died from cardiovascular causes; neither was related to the device. In addition, 19 patients (25.7%) died from other causes during follow-up, a median of 2 [1-2.6] years after the LAAC. Noncardiovascular deaths are analyzed in detail in table 1 of the supplementary data (figure 3).
Characteristics of major bleeding events (BARC ≥ 3a) occurring during follow-up
| Type of bleeding | BARC | Repeated bleeding | Same type of repeated bleeding | Time after LAAC, mo | SAPT during repeated bleeding | HAS-BLED | Death | Time until death after bleeding, mo |
|---|---|---|---|---|---|---|---|---|
| 1 Gastrointestinal | 3b | Yes | Yes | 47 | No | 5 | Yes | 8 |
| 2 Gastrointestinal | 3a | Yes | No | 16 | Yes | 6 | No | — |
| 3 Gastrointestinal | 3a | Yes | Yes | 24 | No | 4 | Yes | 10 |
| 4 Gastrointestinal | 3a | Yes | Yes | 1 | Yes | 5 | Yes | 13 |
| 5 Unknown | 3a | No | — | 9 | No | 4 | No | — |
| 6 Gastrointestinal | 3a | Yes | Yes | 1 | Yes | 5 | No | — |
| 7 Gastrointestinal | 3a | Yes | Yes | 6 | Yes | 4 | Yes | 14 |
| 8 Unknown | 3a | Yes | No | 0 | Yes | 5 | No | — |
| 9 Gastrointestinal | 3a | Yes | Yes | 27 | Yes | 6 | No | — |
| 10 Cerebral | 3c | No | — | 1 | Yes | 5 | No | — |
| 11 Gastrointestinal | 3b | Yes | Yes | 6 | Yes | 5 | No | — |
| 12 Gastrointestinal | 3b | Yes | Yes | 4 | Yes | 5 | Yes | 1 |
BARC, Bleeding Academic Research Consortium; LAAC, left atrial appendage closure; SAPT, single antiplatelet therapy.
The results of this study, which assessed the safety and effectiveness of a SAPT strategy after LAAC, can be summarized in 3 key points:
- 1.
These patients had a very low rate of ischemic and embolic events after a median follow-up of 2.5 years.
- 2.
The incidence of DRT with the use of SAPT was similar to that of other studies and had no clinical impact on embolisms.
- 3.
Bleeding events were reduced by 2.3% in absolute terms and by 26% vs the theoretical risk of bleeding.
Antithrombotic therapy after LAAC is prescribed to avoid DRT and, consequently, the onset of new systemic embolic events. However, the biological process of intracardiac device endothelialization is not completely understood and the time required to complete the endothelialization process is unknown.20,21
Both the incidence and clinical consequences (embolisms) of DRT after LAAC vary widely among the published series. The most recent studies report annual incidences of 0.9% to 7.2%.21 The largest European registry, published by the EWOLUTION researchers with the WATCHMAN device,12 found a DRT incidence (identified by TEE or CT) of 4.1% (34 of 835 patients); 91% of cases were detected before 90 days after implantation (median, 54 days). None of the patients with DRT developed thromboembolic events such as stroke/transient ischemic attack or peripheral embolism during a 21-month follow-up. Similarly, in the Amplatzer Cardiac Plug retrospective multicenter study,22 the incidence of DRT (diagnosed by TEE) was 3.2% and was not associated with thromboembolic events.
This contrasts with the study published by Dukkipati et al.,23 in which the authors retrospectively analyzed the patients included in the PROTECT-AF, PREVAIL, CAP, and CAP2 pilot studies. All cases of DRT were diagnosed using TEE with high protocol adherence (90.9%-98.7%). Among a total of 1739 patients, DRT was found in 65 (3.74%). The proportion of systemic embolism was higher in the DRT group: 6.28 vs 1.65/100 patient-years (P<.001). Multivariate analysis revealed the following predictors of DRT: history of stroke/transient ischemic attack, permanent AF, left atrial appendage diameter, and left ventricular ejection fraction. Along these lines are the study results obtained by Simard et al.24 on predictive factors for DRT. The authors of the study performed a matched case-control analysis with and without DRT in a 1:2 proportion with various LAAC devices. DRT was associated with a higher incidence of major cardiovascular events during follow-up (29.5% vs 14.4%; P<.001), particularly ischemic stroke (16.9% vs 3.6%; P=.01). No effect was found for the antiplatelet therapy regimen prescribed after LAAC (SAPT vs DAPT) on DRT onset. Two main predictors of DRT were identified—hypercoagulability and the presence of pericardial effusion—and several minor predictors: renal failure, implantation depth >10mm from the pulmonary veins, and nonparoxysmal AF. The presence of 1 major risk factor or 2 minor factors conferred a 2.1-fold increased risk of DRT during follow-up.
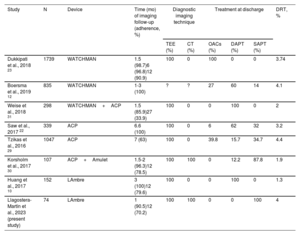
Regarding the LAmbre device, very low rates of DRT have been reported during follow-up, between 0% and 1.3% at 12-months of follow-up with standard DAPT (for at least 3 months).10,25,26 Our study is the first to show DRT data for this type of device with the use of SAPT.
In our study, the DRT incidence was 1.5% at 30 days after LAAC and 3.85% at 12 months, which represented 4% of the total cohort and 2.5% per TEE performed. Although this incidence of DRT is higher than that previously described with the LAmbre device, these studies were performed in very small populations (n=11-24),26,27 with DRT follow-up times of up to 3 months,10 or with low rates of echocardiographic monitoring at 12 months of follow-up (60%).25
In general, we believe that this result is promising, considering that all of the above-mentioned DRT studies were performed with OACs or, typically, DAPT. The low incidence of DRT in our study could be explained by chance, given the relatively small cohort; however, we believe that the design of the LAmbre device and the material used, together with the implantation technique, facilitated a complete closure of the appendage and that this can promote the rapid endothelialization of the device.25,28Table 5 summarizes the main results for DRT from the above-mentioned studies.
Comparison of DRT incidence by device, diagnostic method, and antithrombotic therapy
| Study | N | Device | Time (mo) of imaging follow-up (adherence, %) | Diagnostic imaging technique | Treatment at discharge | DRT, % | |||
|---|---|---|---|---|---|---|---|---|---|
| TEE (%) | CT (%) | OACs (%) | DAPT (%) | SAPT (%) | |||||
| Dukkipati et al., 2018 23 | 1739 | WATCHMAN | 1.5 (98.7)6 (96.8)12 (90.9) | 100 | 0 | 100 | 0 | 0 | 3.74 |
| Boersma et al., 2019 12 | 835 | WATCHMAN | 1-3 (100) | ? | ? | 27 | 60 | 14 | 4.1 |
| Weise et al., 2018 31 | 298 | WATCHMAN+ACP | 1.5 (85.9)27 (33.9) | 100 | 0 | 0 | 100 | 0 | 2 |
| Saw et al., 2017 22 | 339 | ACP | 6.6 (100) | 100 | 0 | 6 | 62 | 32 | 3.2 |
| Tzikas et al., 2016 29 | 1047 | ACP | 7 (63) | 100 | 0 | 39.8 | 15.7 | 34.7 | 4.4 |
| Korsholm et al., 2017 30 | 107 | ACP+Amulet | 1.5-2 (96.3)12 (78.5) | 100 | 100 | 0 | 12.2 | 87.8 | 1.9 |
| Huang et al., 2017 10 | 152 | LAmbre | 3 (100)12 (79.6) | 100 | 0 | 0 | 100 | 0 | 1.3 |
| Llagostera-Martín et al., 2023 (present study) | 74 | LAmbre | 1 (90.5)12 (70.2) | 100 | 100 | 0 | 0 | 100 | 4 |
ACP, Amplatzer Cardiac Plug; CT, computed tomography; DAPT, dual antiplatelet therapy; DRT, device-related thrombosis; SAPT, single antiplatelet therapy; TEE, transesophageal echocardiography.
In the present study, the observed rate of stroke was 0.5%/y, which indicates a relative risk reduction of 91% vs the expected rate of events according to the CHA2DS2-VASc score. This is a highly pertinent result because, first, it shows that the rate of ischemic cerebral events in patients with SAPT after LAAC is extremely low and, second, it is comparable with the results of other studies that used more aggressive antithrombotic therapy regimens after LAAC. In the EWOLUTION study12 (which included 1020 patients with LAAC treated with a WATCHMAN device and had a 2-year follow-up), 60% of patients received DAPT after LAAC and 27% were treated with OACs for at least 2 months. During follow-up, the annual rate of ischemic stroke was 1.3% and the risk reduction was 83%. In addition, the European Amplatzer registry29 included 1047 patients followed for an average of 13 months. Overall, 59% of the patients were discharged with OACs or DAPT after the LAAC (mean duration, 3.8 months). In this case, the annual rate of stroke/transient ischemic attack was reported to be 2.3% and the risk reduction was 59%. Finally, if we consider the initial descriptive study of the LAmbre device in Europe, Park et al.25 reported a rate of cerebral ischemic events of 2%/y with a 3-month duration of DAPT.
As far as we know, only 1 study has assessed the use of SAPT after LAAC with the ACP and Amulet devices in a population with high bleeding risk.30 In that nonrandomized study, Korsholm et al. included 107 patients who were prescribed aspirin monotherapy after the procedure. Both the study population and the results are comparable to those of our cohort. The cohort had a mean CHA2DS2-VASc score of 4.4±1.6 and a HAS-BLED of 4.1±1.1. The reported stroke incidence was 2.3%/y during follow-up while the relative risk reduction was 61%.
Bleeding eventsDespite the reduction in antithrombotic therapy after LAAC, we still detected a high rate of major bleeding events during follow-up (12 patients, 16.2%; 6.4%/y). Because 3 patients were not taking SAPT at the time of the event (25% of bleeding events), their events cannot be considered related. This strengthens the theory that the population exhibits very high bleeding risk and a propensity for bleeding events, even without any antithrombotic therapy. However, the rates are clearly lower if the bleeding events in our population are compared with the theoretical bleeding risk according to the HAS-BLED scale. The theoretical incidence of bleeding according to HAS-BLED score would be 8.7%/y, which represents a relative risk reduction of 26.4% vs the actual incidence of bleeding events.
Our results agree with those of other studies analyzing the presence of bleeding events in real-life clinical practice and with shorter DAPT regimens after LAAC. In the above-mentioned 2-year subanalysis of the EWOLUTION study,12 a 2.7%/y rate of bleeding events was found in the total cohort of 1020 patients, with a relative risk reduction vs bleeding expected with HAS-BLED of 46%. If the subgroup of patients with bleeding history is analyzed, the incidence of events increases to 4.5%/y and the relative risk reduction is 30%.
The only other study to assess the SAPT strategy, by Korsholm et al.,30 found a major bleeding rate of 3.8%/y, with a relative risk reduction of 57% vs that expected with HAS-BLED. Although the population of that study is comparable to our population in terms of bleeding risk by HAS-BLED (mean, 4.1), the populations differ regarding other comorbidities, such as chronic kidney disease (13% vs 72% in our registry), which are directly related to the platelet dysfunction and altered hemostasis.32 This could explain our more discrete reduction in major bleeding vs Korsholm et al.30
LimitationsThe main limitations of the present study are due to its observational and nonrandomized design. The results are limited by the lack of a control group or alternative treatment validating the findings. In the absence of a control group, event prediction estimates of the CHA2DS2-VASc and HAS-BLED scales were used as an approximation. In addition, in our study, the treating physicians established the SAPT duration; studies are required to determine its optimal duration. Moreover, the sample size and TEE follow-up rate limit the interpretation of the results. The study was performed with a single device. Finally, the sample was drawn from a single center and its size is relatively small. Larger series and randomized experimental studies are required to confirm these promising findings.
CONCLUSIONSA SAPT regimen after LAAC with the LAmbre device appears to be a safe and effective option in patients with very high thromboembolic and bleeding risk. Although the optimal duration remains to be determined, the simplified antiplatelet therapy after LAAC evaluated in our study represents a change to the current paradigm and is an attractive option from the clinical perspective. Further studies corroborating our findings are required.
- –
Most patients undergoing LAAC have a very high bleeding risk that limits subsequent antithrombotic therapy. Current clinical practice guidelines recommend DAPT of variable durations, although these recommendations are based on slight evidence. Device thrombosis is an infrequent event during the follow-up of these patients.
- –
A SAPT regimen after LAAC with the LAmbre device seems to be safe and effective, with low rates of device thrombosis and embolic events during follow-up of a cohort with considerable comorbidity and high ischemic and bleeding risks.
No funding was received for this study.
AUTHORS’ CONTRIBUTIONSM. Llagostera-Martín: study design, patient enrollment, data acquisition, statistical analysis, data interpretation, manuscript drafting, manuscript revision, final approval of the manuscript. M. Cainzos, H. Tizón-Marcos, and A. Calvo-Fernández: data interpretation, manuscript drafting, manuscript revision, final approval of the manuscript. N. Salvatella and H. Cubero-Gallego: patient enrollment, manuscript revision, final approval of the manuscript. A. Mas-Stachurska, A. Sánchez-Carpintero, and L. Molina: data acquisition, manuscript revision, final approval of the manuscript. B. Vaquerizo: study design, data interpretation, manuscript drafting, manuscript revision, final approval of the manuscript.
CONFLICTS OF INTERESTThe authors declare that they have no conflicts of interest related to the content of this article.