Spontaneous coronary artery dissection (SCAD) is an uncommon cause of acute coronary syndrome. The characteristics and in-hospital clinical course of patients with SCAD in Spain remain unknown.
MethodsWe present data from consecutive patients included in the national prospective SCAD registry. Angiographic analysis was performed in a centralized core laboratory.
ResultsBetween June 2015 and April 2019, we included 318 patients with SCAD (358 lesions) from 31 centers. Median age was 53 years, and 88% were women. The most frequent presentation was non–ST-segment elevation acute myocardial infarction (53%). The most frequently involved artery was the left anterior descending coronary artery (44%), predominantly affecting the distal segments (39%) and secondary branches (54%). Most lesions (62%) appeared on angiography as intramural hematoma, without double lumen. Conservative management was selected as the initial approach in most patients (78%). During the index admission, 6% of patients had a major adverse event and 4 patients (1.3%) died. Independent predictors of adverse events were initial management with percutaneous coronary intervention (OR, 5.97; P=.004) and angiographic presentation as intramural hematoma (OR, 4.96; P=.028).
ConclusionsIn Spain, SCAD affects mainly middle-aged women. In most patients, the initial management strategy was conservative with excellent in-hospital survival. Initial management with percutaneous coronary intervention and angiographic presentation as intramural hematoma were related to the presence of in-hospital adverse events.
Registered at ClnicalTrials.gov (Identifier: NCT03607981).
Keywords
Spontaneous coronary artery dissection (SCAD) is a known but relatively rare cause of acute coronary syndrome. It can be defined as a separation of the layers of the coronary artery wall due to a noniatrogenic or nontraumatic cause. Two main mechanisms have been proposed to explain the pathogenesis of SCAD. In the first, the primary event would be the formation of a flap or tear in the intima, whereas in the second, the primary event would be hemorrhage in the media without intimal rupture. This initial aggression would lead to the formation of an intramural hematoma (IHM) that could compress the true arterial lumen and cause myocardial ischemia.1 Although the actual incidence of SCAD is unknown, the number of cases reported has increased, particularly in the last decade, and the profile of patients has also changed.2 Women younger than 50 years now account for a quarter of all patients with SCAD.3 SCAD was first described almost 9 decades ago and most of the evidence published in the first 80 years was based on case reports and small case series. Our understanding of this rare condition, however, has been greatly enhanced in the last decade by data from large, mostly retrospective and non-European, registries of patients with SCAD.4–7 The new evidence led to the recent publication of a position paper on SCAD by the European Society of Cardiology8 and a scientific statement by the American Heart Association.9
As SCAD is a sporadic and rare condition, further evidence is unlikely to emerge from clinical trials and it is therefore essential to conduct observational studies to generate new information. We created a prospective national registry to record all incident cases of SCAD in Spain. In this article, we describe the clinical and angiographic characteristics of the first 318 patients included in this registry, together with details of their treatment and in-hospital progress.
MethodsA prospective registry was designed to consecutively record incident cases of SCAD in hospitals throughout Spain. The design included preparation of a protocol, a case report form, and an informed consent template approved by the ethics committee at the local coordinating center (which later became the national coordinating center). The project was registered in the international ClinicalTrials.gov database (NCT03607981) and endorsed by the Cardiac Catheterization and Interventional Cardiology Section of the Spanish Cardiology Society. It was presented for the first time at the annual meeting of the section in June 2015. The registry is not audited and hospitals throughout Spain were invited to voluntarily participate in the project. All included patients are required to provide signed informed consent.
Clinical and follow-up dataBaseline demographic characteristics, personal history, admission data, and in-hospital and follow-up data were prospectively recorded by assigned personnel at each hospital using a single, purpose-designed case report form.
Angiographic analysisAll coronary angiograms were jointly reviewed by 2 experts (M García-Guimaraes and F Alfonso) at the coordinating center. Cases for which a diagnosis of SCAD could not be confirmed with any degree of certainty after analysis of the angiograms and accompanying information from the hospital of origin were excluded as it was considered highly likely that the patient had a condition other than SCAD. These doubtful cases were also reviewed by a third expert (T Bastante). Angiographic data were systematically recorded using a standardized form containing sections for lesion location, morphologic features, characteristics identified by visual assessment, main characteristics, treatments, and percutaneous coronary intervention (PCI) outcomes.
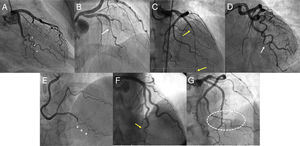
DefinitionsAngiographic patterns were classified using the angiographic SCAD classification system described by Saw et al.10 Lesions with a double-lumen appearance (figure 1A) were classified as type 1 lesions. Lesions displaying diffuse narrowing (>20mm) without a double-lumen appearance were classified as type 2 lesions and further classified into type 2a when the lumen distal to the dissection had a normal diameter (figure 1B) or type 2b when the lesion extended to the distal tip of the artery, without recovery of normal arterial diameter (figure 1C). Lesions with more focal narrowing (<20mm), which can resemble atherosclerotic lesions (figure 1D), were classified as type 3 lesions, and lesions not fitting any of the above patterns (ie, lesions showing an abrupt occlusion without proximal involvement) were classified as type 4 lesions. The morphologic descriptions of Motreff et al.11 were used to identify other angiographic patterns and included the stick insect (figure 1E) and the radish (figure 1F).11 Additional features indicative of SCAD were also considered, such as the broken line pattern (figure 1G).4 Coronary tortuosity was evaluated using the system described by the Mayo Clinic.6 PCI success was determined using the definitions of conventional and SCAD-specific success according to Tweet et al.12 Conventional success refers to a final Thrombolysis in Myocardial Infarction flow grade 2 to 3 (TIMI 2-3) with residual stenosis <30% after stent or scaffold implantation or <50% after simple balloon angioplasty. CAD-specific success refers to an improvement in baseline flow of ≥1 grade to reach TIMI 2-3. Analysis of the presence and characteristics of extracoronary vascular abnormalities (EVAs) was guided by the work of Prasad et al.13 Fibromuscular dysplasia (FMD) was classified as multifocal (sequential areas of stenosis separated by areas of dilatation forming the classic string-of-beads pattern) or unifocal (focal lesions with a tubular appearance). Aneurysm was defined as a ≥50% increase in diameter with respect to the diameter of the normal adjacent segment of the artery. Dilatation was defined as an increase in diameter of <50%. Dissection was defined as a double-lumen morphology in an arterial segment. A composite in-hospital major adverse event (MAE) endpoint was predefined and included all-cause mortality, nonfatal reinfarction, unplanned revascularization, ventricular arrhythmia, heart failure, and stroke. Reinfarction was defined using the third universal definition of myocardial infarction,14 which was the definition in use when the study protocol was written.
Characteristic angiographic findings in patients with spontaneous coronary artery dissection. A: double-lumen image in marginal branch (asterisks). B: possible intramural hematoma (type 2a lesion) (white arrow), with recovery of normal artery diameter distal to the lesion. C: long intramural hematoma (type 2b lesion) (within yellow arrows) in anterior descending artery extending to the distal tip of the coronary artery. D: focal type 3 lesion in marginal branch (white arrow). E: hematoma in distal segment of posterior descending artery with stick image morphology (+). F: hematoma in distal segment of anterior descending artery with radish morphology (yellow arrow). G: intramural hematoma in marginal branch with correction of normal coronary angle, causing the appearance of a broken line (shown in ellipse).
Quantitative variables are expressed as mean±standard deviation or median [interquartile range], and categorical variables as frequency (percentage). A logistic regression model was built to determine factors associated with the occurrence of MAE during hospital admission. Eighteen potential predictors based on clinical criteria and previous evidence were included. Factors significantly associated with MAE in the univariate analysis (P<.20) were included in a multivariate model. For the rest of the analyses, a P value of less than .05 was considered significant. All tests were performed in STATA 12 (StataCorp LLC, United States).
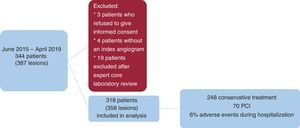
ResultsIn total, 344 patients with 387 lesions treated at 31 hospitals were included in the registry between June 2015 and April 2019. Three patients were excluded because they did not provide informed consent and 4 because the index angiogram was not available. An additional 19 patients were excluded following the expert review at the core laboratory, as it was considered highly likely that they had a diagnosis other than SCAD. In total, 318 patients with 358 lesions were analyzed (figure 2). The 31 hospitals performed 216 897 coronary angiograms between June 2015 and April 2019. The incidence of SCAD in these hospitals was therefore 1.4 cases per 1000 diagnostic angiograms during this period ().
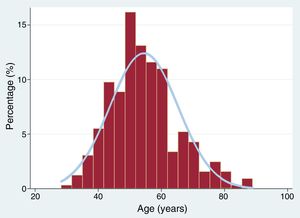
The baseline characteristics of the patients, 88% of whom were women, are shown in table 1. Median age was 53 [47-60] years; the distribution of patients by age group is shown in figure 3. Seventy-eight percent of patients had at least 1 cardiovascular risk factor. The most common factors were a history of smoking (43%), high blood pressure (37%), and hypercholesterolemia (35%). Chronic inflammatory diseases and collagen disorders were very uncommon, affecting just 4% and 1% of patients respectively. Depression and anxiety, by contrast, were relatively common (20% and 17%, respectively). Thirteen percent of patients had a history of hypothyroidism, although just 5% (15 patients) were hypothyroid at the time of SCAD diagnosis.
Patients’ clinical characteristics
| Patients | 318 |
| Women | 279 (88) |
| Age, y | 53 [47-60] |
| Weight, kg | 68 [60-79] |
| Height, cm | 162 [158-167] |
| Race | |
| White | 290 (91) |
| Other | 28 (9) |
| Cardiovascular risk factors | |
| Smoking | |
| Smoker | 88 (28) |
| Exsmoker | 49 (15) |
| Hypertension | 118 (37) |
| Hypercholesterolemia | 111 (35) |
| Diabetes mellitus | 16 (5) |
| Family history of ischemic heart disease | 37 (12) |
| Family history of SCAD | 2 (1) |
| Relevant history | |
| Previous diagnosis of ischemic heart disease | 17 (5) |
| Confirmed previous diagnosis of SCAD | 9 (3) |
| Previous stroke | 12 (4) |
| Ischemic | 11 (3) |
| Hemorrhagic | 1 |
| Connective tissue disease | 2 (1) |
| Inflammatory bowel disease | 14 (4) |
| Depression | 65 (20) |
| Anxiety | 55 (17) |
| History of hypothyroidism | 42 (13) |
| Gynecologic/obstetric events, No. | 279 |
| Menopause | 156 (56) |
| Age at menopause, y | 49±4 |
| Hormone replacement therapy | 15 (5) |
| Oral contraceptives | 22 (8) |
| Intrauterine device | 4 (1) |
| Nulliparous | 38 (14) |
| Multiparous | 148 (53) |
| Miscarriage | 45 (16) |
SCAD, spontaneous coronary artery dissection.
Values are expressed as No. (%), mean±standard deviation, or median [interquartile range].
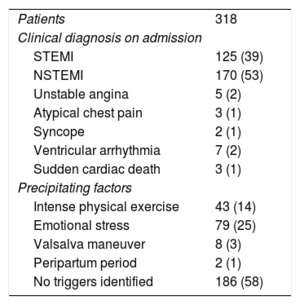
Hospital admission variables are summarized in table 2. The most common presenting condition was non–ST-segment elevation acute myocardial infarction (53% of cases) followed by acute ST-segment elevation myocardial infarction (39% of cases). Ventricular arrhythmia (7%) and sudden cardiac death (3 patients) were uncommon. Potential triggers were identified in 42% of cases, the most common being emotional stress (25%) and intense physical exercise (14%). Just 2 cases of SCAD occurred during the peripartum period.
Characteristics on admission to hospital
| Patients | 318 |
| Clinical diagnosis on admission | |
| STEMI | 125 (39) |
| NSTEMI | 170 (53) |
| Unstable angina | 5 (2) |
| Atypical chest pain | 3 (1) |
| Syncope | 2 (1) |
| Ventricular arrhythmia | 7 (2) |
| Sudden cardiac death | 3 (1) |
| Precipitating factors | |
| Intense physical exercise | 43 (14) |
| Emotional stress | 79 (25) |
| Valsalva maneuver | 8 (3) |
| Peripartum period | 2 (1) |
| No triggers identified | 186 (58) |
NSTEMI, Non–ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction.
Values are expressed as No. (%).
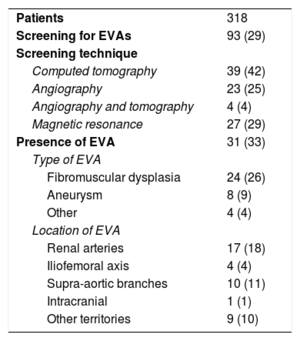
Screening for EVAs was performed in 93 patients (29% of the cohort) and abnormalities were detected in 31 of these (33% of those screened) (table 3). The most common abnormality detected was FMD (26%), which affected the renal arteries in 18% of cases and the supra-aortic branches in 11%. The main screening techniques used were computed tomography (46%), conventional invasive angiography (29%), and magnetic resonance angiography (29%).
Screening for EVAs
| Patients | 318 |
| Screening for EVAs | 93 (29) |
| Screening technique | |
| Computed tomography | 39 (42) |
| Angiography | 23 (25) |
| Angiography and tomography | 4 (4) |
| Magnetic resonance | 27 (29) |
| Presence of EVA | 31 (33) |
| Type of EVA | |
| Fibromuscular dysplasia | 24 (26) |
| Aneurysm | 8 (9) |
| Other | 4 (4) |
| Location of EVA | |
| Renal arteries | 17 (18) |
| Iliofemoral axis | 4 (4) |
| Supra-aortic branches | 10 (11) |
| Intracranial | 1 (1) |
| Other territories | 9 (10) |
EVA, extracoronary vascular abnormality.
Values are expressed as No. (%).
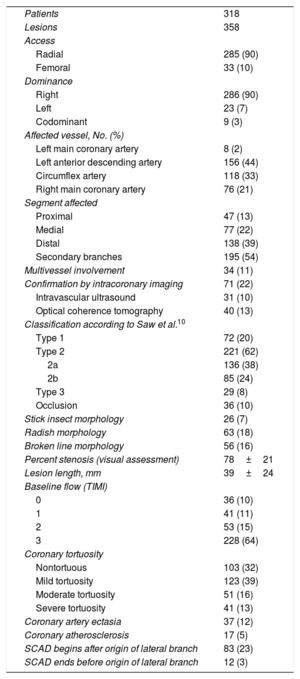
The angiographic patterns detected are summarized in table 4. Coronary angiography was performed using a radial approach in 90% of cases. The main arteries affected were the anterior descending artery (44%) and the circumflex artery (33%). SCAD mostly affected distal vascular territories (39%) and secondary branches (54%). Proximal involvement was observed in just 13% of cases and 11% of patients had multivessel involvement. Intracoronary imaging via intravascular ultrasound or optical coherence tomography was used to confirm the diagnosis of SCAD in 22% of cases. Most lesions (62%) were type 2 according to the Saw SCAD classification system.10 Just 20% had a double-lumen appearance (type 1). Other patterns included the radish (18% of lesions), the broken line (16%), and the stick insect (7%). In 23% of lesions, the SCAD began after the origin (<5mm) of a lateral branch measuring ≥1.5mm. The mean percent stenosis by visual assessment was 78%±21% and mean lesion length was 39±24mm. Baseline TIMI was grade 3 in 64% of cases. Sixty-eight percent of patients met the criteria for coronary tortuosity and 13% had severe tortuosity. Only 5% of lesions were suggestive of atherosclerosis in other coronary segments.
Angiographic characteristics
| Patients | 318 |
| Lesions | 358 |
| Access | |
| Radial | 285 (90) |
| Femoral | 33 (10) |
| Dominance | |
| Right | 286 (90) |
| Left | 23 (7) |
| Codominant | 9 (3) |
| Affected vessel, No. (%) | |
| Left main coronary artery | 8 (2) |
| Left anterior descending artery | 156 (44) |
| Circumflex artery | 118 (33) |
| Right main coronary artery | 76 (21) |
| Segment affected | |
| Proximal | 47 (13) |
| Medial | 77 (22) |
| Distal | 138 (39) |
| Secondary branches | 195 (54) |
| Multivessel involvement | 34 (11) |
| Confirmation by intracoronary imaging | 71 (22) |
| Intravascular ultrasound | 31 (10) |
| Optical coherence tomography | 40 (13) |
| Classification according to Saw et al.10 | |
| Type 1 | 72 (20) |
| Type 2 | 221 (62) |
| 2a | 136 (38) |
| 2b | 85 (24) |
| Type 3 | 29 (8) |
| Occlusion | 36 (10) |
| Stick insect morphology | 26 (7) |
| Radish morphology | 63 (18) |
| Broken line morphology | 56 (16) |
| Percent stenosis (visual assessment) | 78±21 |
| Lesion length, mm | 39±24 |
| Baseline flow (TIMI) | |
| 0 | 36 (10) |
| 1 | 41 (11) |
| 2 | 53 (15) |
| 3 | 228 (64) |
| Coronary tortuosity | |
| Nontortuous | 103 (32) |
| Mild tortuosity | 123 (39) |
| Moderate tortuosity | 51 (16) |
| Severe tortuosity | 41 (13) |
| Coronary artery ectasia | 37 (12) |
| Coronary atherosclerosis | 17 (5) |
| SCAD begins after origin of lateral branch | 83 (23) |
| SCAD ends before origin of lateral branch | 12 (3) |
SCAD, spontaneous coronary artery dissection; TIMI, Thrombolysis in Myocardial Infarction.
Values are expressed as No. (%) or mean±standard deviation.
Details of the first-line treatments are shown in table 5. A conservative approach was chosen in the majority of cases (78%). PCI was used as a primary treatment in 70 patients (22%). None of the patients were treated with myocardial revascularization. The main indications for primary PCI were TIMI 0-1 at baseline (37%) and ischemia at the time of the procedure (30%) (). The most common PCI procedures were drug-eluting stent implantation (58%), simple balloon angioplasty (15%), and bioresorbable device implantation (13%). The PCI success rate was 57% according to the conventional definition and 81% according to the SCAD-specific definition.
Primary treatment and outcomes
| Patients | 318 |
| Primary treatment | |
| Conservative | 248 (78) |
| PCI | 70 (22) |
| Guidance only | 4 (6) |
| Balloon angioplasty | 11 (15) |
| Cutting balloon | 2 (3) |
| Drug-eluting balloon | 2 (3) |
| Conventional stent | 2 (3) |
| Drug-eluting stent | 42 (58) |
| Bioresorbable device | 9 (13) |
| Myocardial revascularization | 0 |
| Conventional PCI success (TIMI flow grade 2-3 and stenosis diameter <30%-50%) | 41 (57) |
| SCAD-specific PCI success (improvement of flow with final TIMI flow grade 2-3) | 58 (81) |
PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection; TIMI, Thrombolysis in Myocardial Infarction.
Values are expressed as No. (%).
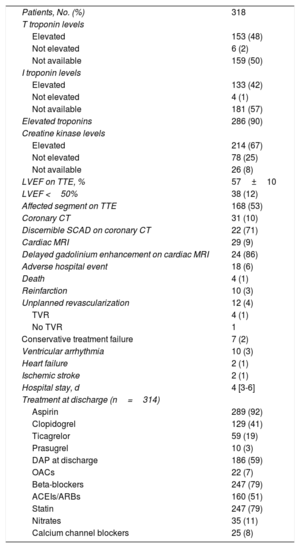
In-hospital events and data and details of treatment on discharge are shown for all patients in table 6. Troponins were elevated in 90% of patients and creatine kinase levels in 67%. Transthoracic echocardiography on admission showed left ventricular dysfunction (left ventricular ejection fraction <50%) in just 12% of patients and segmental abnormalities in 53%. Eighteen patients (6% of the total cohort) experienced an adverse event during hospitalization. Four patients died of refractory cardiogenic shock. Ten patients (3%) met the criteria for reinfarction and 12 (4%) required PCI during hospitalization. Four of these had undergone PCI as first-line therapy and required revascularization of the target vessel due to recurrent ischemia; their angiograms showed signs of late SCAD progression. Seven of the patients treated conservatively at baseline (3% of all patients in this group) required PCI during hospitalization due to recurrent ischemia. Median length of stay was 4 [3-6] days. At discharge, 92% of patients were on low-dose aspirin and more than half (59%) were on dual antiplatelet therapy, although relatively few were on potent antiplatelet agents (ticagrelor, 19% and prasugrel, 3%). Additional treatments at the time of discharge included beta-blockers (79%), statins (79%), and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (51%).
Hospitalization events and outcomes
| Patients, No. (%) | 318 |
| T troponin levels | |
| Elevated | 153 (48) |
| Not elevated | 6 (2) |
| Not available | 159 (50) |
| I troponin levels | |
| Elevated | 133 (42) |
| Not elevated | 4 (1) |
| Not available | 181 (57) |
| Elevated troponins | 286 (90) |
| Creatine kinase levels | |
| Elevated | 214 (67) |
| Not elevated | 78 (25) |
| Not available | 26 (8) |
| LVEF on TTE, % | 57±10 |
| LVEF <50% | 38 (12) |
| Affected segment on TTE | 168 (53) |
| Coronary CT | 31 (10) |
| Discernible SCAD on coronary CT | 22 (71) |
| Cardiac MRI | 29 (9) |
| Delayed gadolinium enhancement on cardiac MRI | 24 (86) |
| Adverse hospital event | 18 (6) |
| Death | 4 (1) |
| Reinfarction | 10 (3) |
| Unplanned revascularization | 12 (4) |
| TVR | 4 (1) |
| No TVR | 1 |
| Conservative treatment failure | 7 (2) |
| Ventricular arrhythmia | 10 (3) |
| Heart failure | 2 (1) |
| Ischemic stroke | 2 (1) |
| Hospital stay, d | 4 [3-6] |
| Treatment at discharge (n=314) | |
| Aspirin | 289 (92) |
| Clopidogrel | 129 (41) |
| Ticagrelor | 59 (19) |
| Prasugrel | 10 (3) |
| DAP at discharge | 186 (59) |
| OACs | 22 (7) |
| Beta-blockers | 247 (79) |
| ACEIs/ARBs | 160 (51) |
| Statin | 247 (79) |
| Nitrates | 35 (11) |
| Calcium channel blockers | 25 (8) |
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; CT, computed tomography; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; OACs, oral anticoagulants; SCAD, spontaneous coronary artery dissection; TTE, transthoracic echocardiogram; TVR, target vessel revascularization.
Data are expressed as No (%) or mean±standard deviation.
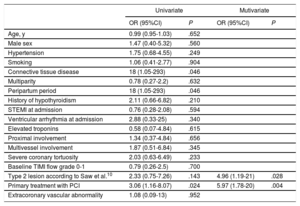
The results of the univariate and multivariate analyses of predictors of in-hospital MAE are shown in table 7. The following variables were identified as independent predictors in the multivariate analysis: PCI vs conservative treatment at baseline, with an adjusted odds ratio of 5.97 (P=.004), and angiographic IMH (type 2 lesion in Saw SCAD classification10) with an adjusted odds ratio of 4.96 (P=.028).
Predictors of composite major adverse event during hospitalization
| Univariate | Mutivariate | |||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Age, y | 0.99 (0.95-1.03) | .652 | ||
| Male sex | 1.47 (0.40-5.32) | .560 | ||
| Hypertension | 1.75 (0.68-4.55) | .249 | ||
| Smoking | 1.06 (0.41-2.77) | .904 | ||
| Connective tissue disease | 18 (1.05-293) | .046 | ||
| Multiparity | 0.78 (0.27-2.2) | .632 | ||
| Peripartum period | 18 (1.05-293) | .046 | ||
| History of hypothyroidism | 2.11 (0.66-6.82) | .210 | ||
| STEMI at admission | 0.76 (0.28-2.08) | .594 | ||
| Ventricular arrhythmia at admission | 2.88 (0.33-25) | .340 | ||
| Elevated troponins | 0.58 (0.07-4.84) | .615 | ||
| Proximal involvement | 1.34 (0.37-4.84) | .656 | ||
| Multivessel involvement | 1.87 (0.51-6.84) | .345 | ||
| Severe coronary tortuosity | 2.03 (0.63-6.49) | .233 | ||
| Baseline TIMI flow grade 0-1 | 0.79 (0.26-2.5) | .700 | ||
| Type 2 lesion according to Saw et al.10 | 2.33 (0.75-7.26) | .143 | 4.96 (1.19-21) | .028 |
| Primary treatment with PCI | 3.06 (1.16-8.07) | .024 | 5.97 (1.78-20) | .004 |
| Extracoronary vascular abnormality | 1.08 (0.09-13) | .952 | ||
95%CI, 95% confidence interval; OR, odds ratio; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
We have analyzed data from the first prospective registry of SCAD patients in Spain and the largest collection of cases in Europe to date. Over a period of almost 4 years, data on 318 incident cases of SCAD were consecutively added to the registry by 31 hospitals across Spain. A key strength of this study is the rigorous review of angiograms by a core team of experts at the coordinating center. In addition, diagnosis was confirmed by intracoronary imaging (intravascular ultrasound or optical coherence tomography) in 22% of cases. This rate is much higher than the rate of 7.6% recently reported by Saw et al.5 in the largest prospective cohort study published to date (750 patients). Confirmation of diagnosis is important in SCAD, as diagnosis by angiography alone can be challenging. Although the wide use of intracoronary imaging attests to the diagnostic accuracy of the cases included in our registry, intracoronary diagnostic techniques must be employed with extreme caution in patients prone to coronary artery dissection and should only be used in equivocal cases or to guide PCI in patients with known SCAD.
Our study revealed a number of very interesting findings. First, our results seems to confirm the indication of conservative treatment as first-line therapy for SCAD, supporting previous evidence and recent recommendations.8,9,15 In addition, just 3% of patients treated with a conservative approach required revascularization with PCI during hospitalization. It should be noted, however, that 6% of patients overall experienced adverse events during admission.
Second, our results shed light on the use of PCI in the setting of SCAD. PCI was performed as first-line therapy in 22% of patients and the main indications were poor distal flow in the affected coronary artery and signs of ischemia at the time of the procedure. This rate is higher than the rate of 14% described by Saw et al.5 in the prospective Canadian cohort of 750 patients. PCI also appears to be associated with clearly suboptimal outcomes in SCAD compared with atherosclerotic coronary artery disease. The respective conventional and SCAD-specific success rates of 47% and 81% are very similar to rates reported in other large series of patients with SCAD. Saw et al.5 reported a partial success rate of 41% (residual dissection or stenosis ≤50% with final TIMI 3 or improvement in baseline flow) and a failure rate of 30% for PCI in their prospective cohort study. The Mayo Clinic group also described a SCAD-specific PCI failure rate of 30%.12 PCI as first-line therapy in our cohort was associated with an increased risk of adverse events during hospitalization. This finding, however, should be interpreted with caution, as the choice of PCI over conventional treatment was probably influenced by the patients’ worse risk profile and clinical presentation. In view of the above, it would seem wise to limit PCI to patients more likely to derive greater benefit from percutaneous revascularization than from conservative treatment (ie, patients with total vessel occlusion, proximal and/or left coronary trunk involvement, current or recurrent ischemia, or hemodynamic instability).
Third, visualization of IMH on the angiogram (type 2 lesion in the SCAD classification system) compared with the classic type 1 double-lumen image was independently associated with MAE during admission. This would seem to support the recent findings of Waterbury et al.,16 who reported significant SCAD progression within 14 days of conservative treatment and a higher risk of progression in patients with type 2 vs type 1 lesions. In addition, an intimal tear was observed in the repeat angiogram for 20% of lesions initially showing an IMH pattern only. This progression would appear to corroborate the outside-in theory according to which intramural hemorrhage would be the primary mechanistic event in SCAD, at least in some patients.
Fourth, just 29% of patients in our cohort were screened for EVAs. This rate is remarkably low, particularly if we compare it to the rate of 73% recently reported by Saw et al.5 in Canada. The detection rates in the 2 cohorts, however, were similar (33% for EVAs and 26% for FMD in our cohort vs 31% for FMD in the Canadian cohort), even though magnetic resonance angiography, which is less sensitive than invasive angiography in the detection of FMD,17 was used in almost one-third of patients in our study. These findings highlight the need for greater awareness in Spain of the importance of ruling out EVAs in patients with SCAD, in line with recent expert recommendations.8,9
LimitationsThe main limitation of this study is its observational design, as patients were not randomly assigned to diagnostic or treatment groups. However, this was a large (the largest to date in Europe), prospective, multicenter study of a rare condition that provides insights into the characteristics and treatment of patients with SCAD in real-world settings. Expert review of angiograms by a core laboratory team is the best strategy for overcoming the occasional challenges encountered with diagnosing SCAD by angiography. It should be noted, however, that the lack of sensitivity associated with conventional angiography for the diagnosis of SCAD may have resulted in underdiagnosis and could explain the lack of resolution of cases incorrectly identified at the participating hospitals.
ConclusionsSCAD in Spain mainly affects middle-aged women and associated cardiovascular risk factors are common. Most patients were initially treated with a conservative approach and survival rates from admission to discharge were excellent. Outcomes of PCI as first-line therapy were clearly suboptimal. Very few patients treated conservatively required subsequent treatment with PCI while in hospital. Primary treatment with PCI and the presence of IMH without a double-lumen image on the index angiogram were associated with MAE during hospitalization.
FundingThis project was partly funded by public funds from the Carlos III Health Institute through a Río Hortega contract.
Conflicts of InterestNone declared.
- –
SCAD is a relatively rare cause of acute coronary syndrome. Recent data from registries of patients with SCAD (mostly retrospective and non-European) have improved previous evidence based on case reports and small series of patients.
- –
Data from a prospective national SCAD registry in Spain shows that SCAD mainly affects middle-aged women and that classic cardiovascular risk factors are common. The most common angiographic pattern was IHM without the typical double-lumen image. Conservative treatment was used in most cases and hospital survival rates were excellent. PCI success rates in patients with SCAD were clearly suboptimal. Very few patients who underwent primary conservative treatment required PCI during hospitalization. Primary PCI and IMH without a double-lumen image on the index angiogram were independently associated with adverse events during hospitalization.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.04.002
The participating hospitals and researchers are listed in the