Both cancer treatment and survival have significantly improved, but these advances have highlighted the deleterious effects of vascular complications associated with anticancer therapy. This consensus document aims to provide a coordinated, multidisciplinary and practical approach to the stratification, monitoring and treatment of cardiovascular risk in cancer patients. The document is promoted by the Working Group on Cardio Oncology of the Spanish Society of Cardiology (SEC) and was drafted in collaboration with experts from distinct areas of expertise of the SEC and the Spanish Society of Hematology and Hemotherapy (SEHH), the Spanish Society of Medical Oncology (SEOM), the Spanish Society of Radiation Oncology (SEOR), the Spanish Society of General and Family Physicians (SEMG), the Spanish Association of Specialists in Occupational Medicine (AEEMT), the Spanish Association of Cardiovascular Nursing (AEEC), the Spanish Heart Foundation (FEC), and the Spanish Cancer Association (AECC).
Keywords
Cardiovascular (CV) toxicity induced by hematology-oncology treatments is a growing clinical problem.1,2 Cancer and CV disease are connected by multiple pathophysiologic mechanisms and share risk factors. Their combined treatment is thus challenging and requires a multidisciplinary approach involving cardiologists and hematology-oncology specialists to minimize cardiotoxic effects.3–5 Cardiotoxicity is defined as any CV event secondary to hematology-oncology treatment.1,2 Heart failure has traditionally attracted the most attention, but with the development of targeted therapies and longer survival times among patients with cancer, vascular toxicity is becoming more common.6 CV disease prevention measures are important in patients with cancer and poor adherence is associated with an increased incidence of CV events.7 To ensure coordinated care provision, both hematology-oncology specialists and patients need to be aware of the importance of monitoring CV risk. The aim of this consensus statement is to provide a practical guide to multidisciplinary strategies for monitoring and controlling CV risk during the different stages of cancer care. The methodology employed is described in (”), which also contains a list of the experts who participated in preparing the consensus statement and the scientific societies they represent ().
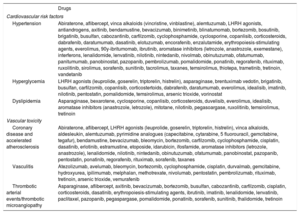
Hematology-Oncology Treatments Associated With Vascular Toxicity and Early Onset of CV Risk FactorsClinicians need to keep abreast of advances in cancer drugs and radiotherapy and their potential toxicities. The drugs associated with vascular toxicity and early onset of CV risk factors are shown in table 1.2,6,8 They are further classified according to their use in different types of cancer in . Radiotherapy has been linked to an increased risk of early atherosclerosis in vascular structures included in the radiation field; the mechanisms involved include endothelial dysfunction, inflammation, and oxidative stress.9–11
Drugs used in cancer patients associated with the development of cardiovascular risk factors or early vascular disease
| Drugs | |
|---|---|
| Cardiovascular risk factors | |
| Hypertension | Abiraterone, aflibercept, vinca alkaloids (vincristine, vinblastine), alemtuzumab, LHRH agonists, antiandrogens, axitinib, bendamustine, bevacizumab, binimetinib, blinatumomab, bortezomib, bosutinib, brigatinib, busulfan, cabozantinib, carfilzomib, cyclophosphamide, cyclosporine, copanlisib, corticosteroids, dabrafenib, daratumumab, dasatinib, elotuzumab, encorafenib, enzalutamide, erythropoiesis-stimulating agents, everolimus, 90y-ibritumomab, ibrutinib, aromatase inhibitors (letrozole, anastrozole, exemestane), interferons, lenalidomide, lenvatinib, nilotinib, nintedanib, nivolmab, obinutuzumab, ofatumumab, panitumumab, panobinostat, pazopanib, pembrolizumab, pomalidomide, ponatinib, regorafenib, rituximab, ruxolitinib, sirolimus, sorafenib, sunitinib, tacrolimus, taxanes, temsirolimus, thiotepa, trametinib, tretinoin, vandetanib |
| Hyperglycemia | LHRH agonists (leuprolide, goserelin, triptorelin, histrelin), asparaginase, brentuximab vedotin, brigatinib, busulfan, carfilzomib, copanlisib, corticostertoids, dabrafenib, daratumumab, everolimus, idealisib, imatinib, nilotinib, pentostatin, pomalidomide, temsirolimus, arsenic trioxide, vorinostat |
| Dyslipidemia | Asparaginase, bexarotene, cyclosporine, copanlisib, corticosteroids, duvelisib, everolimus, idealisib, aromatase inhibitors (anastrozole, letrozole), mitotane, nilotinib, pegasoargase, ruxolitinib, temsirolimus, tretinoin |
| Vascular toxicity | |
| Coronary disease and accelerated atherosclerosis | Abiraterone, aflibercept, LHRH agonists (leuprolide, goserelin, triptorelin, histrelin), vinca alkaloids, aldesleukin, alemtuzumab, pyrimidine analogues (capecitabine, cytarabine, 5 fluorouracil, gemcitabine, tegafur), bendamustine, bevacizumab, bleomycin, bortezomib, carfilzomib, cyclophosphamide, cisplatin, dasatinib, erlotinib, estramustine, etoposide, idarubicin, ifosfamide, aromatase inhibitors (letrozole, anastrozole), lenalidomide, nilotinib, nintedanib, obinutuzumab, ofatumumab, panobinostat, pazopanib, pentostatin, ponatinib, regorafenib, rituximab, sorafenib, taxanes |
| Vasculitis | Atezolizumab, avelumab, bleomycin, bortezomib, cyclophosphamide, cisplatin, durvalmab, gemcitabine, hydroxyurea, ipilimumab, melphalan, methotrexate, nivolumab, pentostatin, pembrolizumab, rituximab, tretinoin, arsenic trioxide, vemurafenib |
| Thrombotic arterial events/thrombotic microangiopathy | Asparaginase, aflibercept, axitinib, bevacizumab, bortezomib, busulfan, cabozantinib, carfilzomib, cisplatin, corticosteroids, dasatinib, erythropoiesis-stimulating agents, ibrutinib, imatinib, lenalidomide, lenvatinib, paclitaxel, pazopanib, pegaspargase, pomalidomide, ponatinib, sorafenib, sunitinib, thalidomide, tretinoin |
LHRH, luteinizing hormone-releasing hormone.
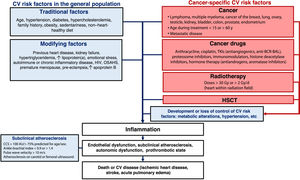
Stratification of CV risk in patients with cancer is crucial to establish risk factor control goals, initiate targeted prevention strategies, and design surveillance and follow-up plans.12 CV risk should thus be assessed periodically throughout the continuum of cancer care.
European guidelines13,14 recommend using the Systemic Coronary Risk Estimation (SCORE) model, whose algorithm includes age, sex, smoking, cholesterol levels, and systolic blood pressure. The usefulness of the SCORE model in patients with cancer was demonstrated in a recent study in which higher scores were associated with higher rates of severe cardiotoxicity and all-cause mortality at 2 years.15
Patients with a history of CV disease, long-standing diabetes, chronic kidney failure, familial hypercholesterolemia, extremely high lipoprotein(a), or subclinical atherosclerosis detected by noninvasive imaging techniques should be classified as high risk or very high risk.14 Key modifiers of risk include a family history of premature CV disease, autoimmune diseases, and human immunodeficiency virus infection14 (figure 1).
CV risk in special populations (individuals aged < 40 years or > 70 years or with diabetes or previous CV disease) can be evaluated using specific calculators available at the European U-Prevent website.16
Current CV risk models for the general population do not include cancer or associated treatments or consider the competing risk of cancer mortality.17 They therefore underestimate CV risk in hematology-oncology patients.18,19
Modifiers of CV riskCancer survivors are 2 to 7 times more likely to die of CV disease than members of the general population.20–22 CV deaths are most common within a year of cancer diagnosis or after a long survival period (U-curve pattern).22–24 Mortality rates are highest among survivors of childhood cancer,25–29 young patients,22,23 patients older than 60 years,22 and patients with metastatic disease.22 Most CV deaths in patients diagnosed with cancer after the age of 40 years occur in the setting of prostate, breast, colorectal, and lung cancer.22 In cancers associated with longer survival times (Hodgkin lymphoma and prostate, bladder, endometrial, thyroid, or testicular cancer23,30,31) CV deaths can outnumber cancer deaths ().
The higher prevalence of CV risk factors in hematology-oncology patients21 is not the only explanation for their higher levels of CV risk. Indeed, certain cancers have been independently linked to the development of CV disease, examples being breast cancer,32 lung cancer,20,32–34 ovarian cancer,20,32 testicular cancer,32 kidney cancer,17,24 and blood malignancies (lymphoma, leukemia, and multiple myeloma20,21,32).
Hematology-oncology treatments can also increase CV risk. Vascular endothelial growth factor inhibitors, for example, are associated with a 3.5-fold increased risk of acute myocardial infarction,35 while second-generation BCR-ABL tyrosine kinase inhibitors (nilotinib, ponatinib, bosutinib) increase the risk of accelerated atherosclerosis, thrombotic events, and peripheral artery disease.36 In multiple myeloma, the use of proteosome inhibitors (carfilzomib), immunomodulators (pomalidomide), and histone deacetylase inhibitors (panobinostat) has been linked to myocardial ischemia.37
Cisplatin has been associated with a 7-fold or higher increased risk of cardiotoxicity in patients with testicular carcinoma.38,39 Finally, bleomycin-etoposide-cisplatin therapy increases the risk of ischemic heart disease, stroke,40,41 and CV death (44% more likely than in the general population).41
RadiotherapyAccelerated atherosclerosis and vascular events are significantly more likely in patients treated with radiotherapy including CV structures in the radiation field (). Patients with lymphoma who receive mediastinal radiotherapy at a dose >30Gy have a 2.5- to 7.3-fold increased risk of CV mortality and a 2.7- to 8.9-fold increased risk of acute myocardial infarction. In survivors of childhood cancer, these risks are increased 5- to 29-fold for CV mortality and 2.4- to 3.6-fold for acute myocardial infarction. Women with breast cancer treated with radiotherapy have a 1.25- to 1.62-fold increased risk of CV mortality compared with the general population. Radiotherapy in patients with lung cancer also increases the risk of cardiac events more than 3-fold. Time from radiotherapy to cardiotoxicity varies according to the type of cancer (table 2). Radiotherapy delivered to the head and neck region or the esophagus is associated with a 1.4- to 5.6-fold increased risk of stroke, while whole-brain radiotherapy used to treat childhood cancer increases the risk of stroke 2.9-fold at doses of 30 to 49Gy and 11-fold at doses >50Gy.
Predisposing factors for CV complications after radiotherapy
| Radiotherapy dose | • 30Gy for mediastinal doses• 5Gy for MHD in children• 20Gy for MHD in lymphoma survivors• >10Gy for MHD in lung cancer |
| Radiotherapy technique | • Orthovoltage or cobalt therapy, 2-dimensional conformal radiotherapy, nonconformal tangential-field radiotherapy• No cardiac protection• Large target volume including heart (mantle field)• Daily fractionation >2Gy• Radiotherapy before 1980 |
| Age | • <21y and >65y |
| Sex | • Increased risk of ischemic heart disease in men• Increased risk of dilated cardiomyopathy in women |
| Tumor location | • Left > right in breast and lung cancer• Increased risk of injury to coronary ostia and great vessels with radiotherapy to base of heart |
| Concomitant chemotherapy | • Increased risk of dilated cardiomyopathy and sudden cardiac death with anthracyclines• Increased risk of stroke with alkylating and platinum agents |
| CV risk factors and previous CV disease | • Hypertension (strongest predictor), tobacco use, dyslipidemia, diabetes, obesity, sedentarism• History of any previous heart disease |
| Time since radiotherapy | • For childhood cancer: >20y• For lymphomas: 10y• For breast cancer: 5-10y• For lung cancers: <2y• Risk of CV complications increases proportionally to time |
CV, cardiovascular; MHD, mean heart dose.
The risk of CV events is influenced by both radiation dose and time since administration (table 2). The dose tolerance limits for healthy CV structures are given in . Mediastinal radiotherapy has been found to increase the relative risk of death in survivors of childhood cancer by 60% for every Gy of mean heart dose (MHD). In patients with lymphoma or breast cancer, the excess relative risk of coronary events lies between 7.4% and 16.5% per Gy of MHD. The association between dose and CV events is linear, but no safe minimum dose has been established.42 CV risk remains elevated for 25 to 40 years after radiotherapy and is exacerbated by the presence of classic CV risk factors. More recent radiotherapy techniques, such as 3D conformal radiotherapy, intensity-modulated radiotherapy, volumetric modulated arc therapy, and proton therapy, together with the use of cardiac protection measures such as multileaf collimators, respiratory control, and decubitus positioning, are associated with lower MHDs and consequently lower cardiotoxicity risk29 ().
Hormone therapyAndrogen deprivation therapy induces metabolic changes (eg, weight gain, increased visceral adiposity, insulin resistance, and unfavorable lipid profiles43,44) and increases the risk of CV events, early-onset atherosclerosis, and ischemic heart disease.44,45 This increased risk is most evident in patients treated with abiraterone, combined androgen blockade, or gonadotropin-releasing hormone agonists. It is less clear in the setting of the latter.44
Aromatase inhibitors are associated with an increased risk of ischemic heart disease, while tamoxifen has been linked to thromboembolism.46
Hematopoietic stem cell transplantCompared with controls without cancer, hematopoietic stem cell transplant recipients with cancer have an increased risk of premature CV death (estimated relative risk [RR], 2.3-4), CV disease (estimated RR, 0.6-5.6), and early onset of CV risk factors such as hypertension, diabetes, and dyslipidemia (estimated RR, 7.0-15.9).47–49 The risk is even higher in the settings of allogeneic hematopoietic stem cell transplant and graft-versus-host disease.
Impact of frailty on decision makingFrailty is a dynamic, age-related, clinical process associated with a loss of physiological reserves in situations of stress; it therefore increases vulnerability and risk of complications.50 Over 50% of older cancer patients are frail or prefrail and have a higher prevalence of risk factors for CVD,51 increasing the likelihood of postoperative complications and poor tolerance of hematology-oncology treatments.52 Frailty assessments in older adults can guide adjustments of CV risk factor control goals to comorbidities and prognosis.13,53,54
Classifying geriatric patients as robust, prefrail, or frail helps in the adjustment of treatment goals and reduces the risk of overtreating frail patients or undertreating stronger ones.55 A range of scales exist for assessing frailty, including the FRAIL scale (table 3) and the more specific Geriatric Assessment in Hematology scale.56
Frailty assessment: the FRAIL scale
| Item | Assessment | Answer | ||
|---|---|---|---|---|
| 1 | Fatigue | Do you feel tired most of the time? | Yes | No |
| 2 | Resistance | Can you walk up a flight of stairs alone without resting and without the use of aids? | Yes | No |
| 3 | Ambulation | Can you walk 100 m alone without resting and without the use of aids? | Yes | No |
| 4 | Presence of ≥5 of the following comorbidities: | Arthritis, diabetes, angina/infarction, hypertension, stroke, asthma, chronic bronchitis, emphysema, osteoporosis, colorectal cancer, skin cancer, depression/anxiety, dementia, leg ulcers | Yes | No |
| 5 | Weight loss | Weight loss >5% in past year | Yes | No |
The FRAIL scale provides a simple means of assessing frailty. Score 1 point for each “yes” in questions 1, 4, and 5 and each “no” in questions 2 and 3. A patient is considered frail if the total score is ≥ 3, prefrail if it is 1-2, and robust if it is 0.
It is also useful to analyze comorbidities in this setting due to their potential impact on decisions regarding control and treatment strategies. Again, a number of scales exist, including the Cumulative Illness Rating Scale-Geriatrics.57
CV risk factor control goalsThe goals for controlling CV risk factors in patients with cancer are the same as those indicated for patients without cancer in current clinical guidelines.13,14,53,54 Apart from assessing past and present history of cancer, it is essential to assign each patient to the correct risk category. It is also important to engage patients in achieving their goals by informing them of their CV risk and targets. The specific goals for patients with cancer are summarized in table 4.13,14,53,54
Cardiovascular risk factor control goals in patients with cancer13,14,53,54
| Blood pressure | <130/80mmHg assuming the treatment is well tolerated; <140/80 in patients ≥65y, avoiding hypotension in all cases | |
| Lipids | Very high risk (SCORE ≥10%) (primary or secondary prevention) | LDL-C <55 mg/dLNon–HDL-C <85 mg/dL |
| High risk (SCORE ≥5 and <10%) | LDL-C <70 mg/dLNon-HDL cholesterol <100 mg/dL | |
| Moderate risk (SCORE ≥1 and <5%) | LDL-C <100 mg/dLNon–HDL-C <130 mg/dL | |
| Low risk (SCORE <1%) | LDL-C <116 mg/dL | |
| Glycemic profile | Glycated hemoglobin <7%<8% in elderly or frail patients or patients with a reduced life expectancy and/or multiple comorbidities | |
| Tobacco use | Absolutely no tobacco intake in any form | |
| Alcohol | Unless contraindicated because of association with cancer or interactions with cancer treatment, maximum of 20μg/d for men and 10g/d for women | |
| Exercise | At least 150 min/wk (30min 5 times a week) of moderate exercise combining aerobic and resistance trainingWhere possible, inclusion of high-risk patients in cardiac rehabilitation programs during and after hematology-oncology treatment | |
| Diet | Mediterranean diet based on whole grains, vegetables, fruit, poultry, fish, extra virgin olive oil, walnutsNo saturated fats, red or processed meat, simple or fast-digesting carbohydrates, or processed foods | |
| Weight | Body mass index of 20-25kg/m2 and waist circumference <94cm for men and <80cm for women | |
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
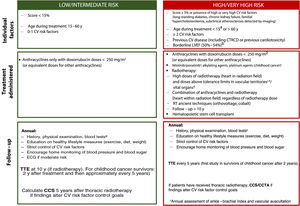
CV disease, alongside cancer recurrence and second cancers, is the main cause of death in patients who have completed hematology-oncology treatment.21 Patients with cancers associated with longer survival are more likely to develop CV disease.23 The higher mortality rates in this setting are due to the complex interaction between CV disease and risk factors (often numerous) and the deleterious effects of hematology-oncology treatment. Evidence in this field, however, is sparse and current follow-up recommendations are mostly based on expert consensus. Early detection of ischemic heart disease, ventricular dysfunction, and vascular disease is necessary to ensure early treatment and improve prognosis.1,2,58
Individual management strategies will vary according to estimated CV risk (figure 2). An annual clinical evaluation with blood tests, including lipid and glycemic profiles, is recommended for cancer survivors with a low CV risk and no symptoms. A periodic electrocardiogram should also be performed in patients at higher risk. Quantification of coronary calcium (calcium score) and coronary computed tomography angiography could be useful for reassessing CV risk and optimizing control strategies, although evidence is lacking in the setting of cancer.
Risk stratification and management after hematology-oncology treatment. CCS, coronary calcium score; CCTA, coronary computed tomography angiography; CTRCD, cancer therapy-related cardiac dysfunction; CV, cardiovascular; ECG, electrocardiogram; LVEF, left ventricular ejection fraction; RT, radiotherapy; TTE, transthoracic echocardiogram. aCV risk in people diagnosed with cancer during childhood can be assessed using the CV risk calculator for childhood cancer survivors, which estimates the risk of ventricular dysfunction, ischemic heart disease, and stroke. Patients are classified as low risk if all the scores indicate low risk (< 3) and as high risk if any of the scores indicate moderate risk (≥ 3). bPatients with a borderline left ventricular ejection fraction are considered high risk if they are being treated with anthracyclines or thoracic radiotherapy including the heart in the radiation field, even at doses not considered high risk. cThese drugs increase the risk of peripheral artery disease. dSee for information on dose tolerance limits for healthy organs according to radiation field. eComplete blood count, basic biochemical profile (creatinine, glomerular filtration rate, liver profile), lipids, and glycated hemoglobin.
Patients who develop signs or symptoms of CV disease, show abnormal test results, or do not achieve adequate control of risk factors should be referred for a CV assessment, preferably at a cardiology-hematology-oncology (CHO) unit.1,2,58
CV Risk Factor Control RecommendationsLifestyleDietFollowing a Mediterranean diet can help control CV risk in both cancer survivors and patients with active cancer.59,60 This diet consists of extra-virgin olive oil, walnuts, fruit, vegetables, pulses, whole-grain products, poultry, and fish. Refined carbohydrates, red meat, processed meat, and foods high in saturated fats should be avoided.
Physical exercise and sportPatients, and particularly those at high risk of cardiotoxicity, should be encouraged to perform at least 150minutes a week of moderate aerobic exercise combined with resistance training.58 Cardiac rehabilitation and exercise programs before, during, and after hematology-oncology treatment are safe and effective CV prevention strategies.61
Toxic habits- •
Tobacco. Patients must be urged to quit tobacco; nicotine replacement therapy, bupropion, and varenicline can be used.62 Electronic cigarettes are not recommended as safe or effective options for quitting smoking.63 In addition, certain components can modify the metabolism of hematology-oncology drugs and reduce their effectiveness.64
- •
Alcohol. If not completely contraindicated because of the type of cancer or the risk of treatment interactions, alcohol intake should be limited to 20g a day for men and 10g a day for women.65
Overweight and obesity are major risk factors in several cancers. In addition, certain hematology-oncology treatments can favor obesity, possibly increasing the risk of cardiotoxicity.66
Diabetes and cancerKeeping blood glucose levels under control in patients with diabetes and active cancer poses a significant clinical challenge. There is no clear evidence that strict glycemic control improves cancer treatment outcomes, so the CHO team should strive to ensure patients remain asymptomatic and at a low risk of acute decompensation. It should also be noted that certain drugs can have a hyperglycemic effect. Examples are corticosteroids, antiandrogens, platinum, 5-fluorouracil, mammalian target of rapamycin inhibitors, and targeted therapies such as nilotinib67 (table 1).
When managing patients with diabetes, priority should be given to antidiabetic drugs with a proven beneficial effect on CV event reduction.13
HypertensionHypertension is one of the most common comorbidities in cancer survivors and patients with active cancer. Its incidence varies according to age, history of hypertension, and type of cancer and/or treatment2,58,68 (). Strict blood pressure control reduces the risk of heart failure and atrial fibrillation69 and avoids the need to interrupt effective hematology-oncology treatment. Treatment adherence and home measurements are essential for achieving stable blood pressure.68 Weekly monitoring is recommended during the first cycle of treatment and thereafter before each cycle.1,53 Patients should be encouraged to follow a heart-healthy lifestyle, limit their salt intake (<5g/d), 53,68 and avoid nonsteroidal anti-inflammatory drugs.68 Angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists are first-line treatments for hypertension in patients with diabetes, pre-existing heart disease, or a risk of heart failure.1,2 Combined treatment with dihydropyridine calcium channel blockers is especially beneficial for patients treated with vascular endothelial growth factor inhibitors.68 Nondihydropyridine calcium channel blockers (verapamil, diltiazem) are not recommended because of the risk of heart failure and interactions (via cytochrome P450) with many cancer drugs. The addition of mineralocorticoid receptor antagonists53 is recommended in patients with refractory hypertension, who should also undergo potassium level monitoring. Beta-blockers should be favored in patients with known heart failure or a risk of atrial fibrillation or ventricular dysfunction due to cardiotoxicity. Fixed combinations of antihypertensives are recommended as they have been linked to better treatment adherence.70
In other cases, hematology-oncology treatments can lower blood pressure levels, possibly requiring dose adjustments and sometimes even treatment interruption. Early reassessment is important to avoid therapeutic inertia and hypertensive rebound in patients who have completed hematology-oncology treatment or developed tolerance.
DyslipidemiaStatins are the treatment of choice for dyslipidemia. They may potentially protect against cancer and in some cases (prostate, breast, and colorectal cancer) have a favorable impact on prognosis,71,72 although randomized trials are needed to confirm this. Statins have also been linked to a lower incidence of heart failure in patients with breast cancer treated with anthracyclines, although their effect on the reduction of cardiotoxicity has not been demonstrated.73 Ezetimibe is safe in cancer. New PCSK9 inhibitors may reduce low-density lipoprotein cholesterol levels to ≤15mg/dL, and while evidence is lacking for patients receiving hematology-oncology treatment, these levels were not associated with an increased incidence of cancer or cancer progression in the FOURIER trial.74
A number of hematology-oncology treatments cause hypertriglyceridemia. Fibrates are a safe and effective treatment option in this setting ().
When prescribing drugs to treat dyslipidemia or other CV risk factors, it is important to first check online updates on drug-drug interactions (RxList website,75 Electronic Medicines Compendium,76 and Spanish Agency for Medicines and Medical Devices’ drug information center77).
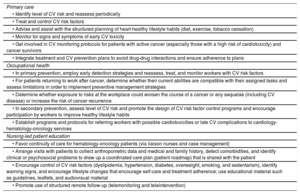
Prevention and health promotion programsPrimary care, occupational health, and nursing teams are key components of CV prevention and health promotion programs throughout the different stages of cancer care.78,79 The involvement of nurses specialized in chronic disease management and care improves health outcomes and continuity of care. Occupational health teams, in turn, can help optimize the management and care of active workers with a history of cancer, leading to improved health outcomes, better continuity and coordination of care, and easier integration and return to work.79
A list of primary care, occupational health, and nursing-led patient education strategies for controlling CV risk factors is provided in table 5.
CV risk factor control strategies for patients with cancer by area: primary care, occupational health, and nursing-led patient education
| Primary care |
| • Identify level of CV risk and reassess periodically |
| • Treat and control CV risk factors |
| • Advise and assist with the structured planning of heart-healthy lifestyle habits (diet, exercise, tobacco cessation) |
| • Monitor for signs and symptoms of early CV toxicity |
| • Get involved in CV monitoring protocols for patients with active cancer (especially those with a high risk of cardiotoxicity) and cancer survivors |
| • Integrate treatment and CV prevention plans to avoid drug-drug interactions and ensure adherence to plans |
| Occupational health |
| • In primary prevention, employ early detection strategies and reassess, treat, and monitor workers with CV risk factors |
| • For patients returning to work after cancer, determine whether their current abilities are compatible with their assigned tasks and assess limitations in order to implement preventive management strategies |
| • Determine whether exposure to risks at the workplace could worsen the course of a cancer or any sequelae (including CV disease) or increase the risk of cancer recurrence |
| • In secondary prevention, assess level of CV risk and promote the design of CV risk factor control programs and encourage participation by workers to improve healthy lifestyle habits |
| • Establish programs and protocols for referring workers with possible cardiotoxicities or late CV complications to cardiology-hematology-oncology services |
| Nursing-led patient education |
| • Favor continuity of care for hematology-oncology patients (via liaison nurses and case management) |
| • Arrange visits with patients to collect anthropometric data and medical and family history, detect comorbidities, and identify clinical or psychosocial problems to draw up a coordinated care plan (patient roadmap) that is shared with the patient |
| • Encourage control of CV risk factors (dyslipidemia, hypertension, diabetes, overweight, smoking, and sedentarism), identify warning signs, and encourage lifestyle changes that encourage self-care and treatment adherence; use educational material such as guidelines, leaflets, and audiovisual material |
| • Promote use of structured remote follow-up (telemonitoring and teleintervention) |
CV, cardiovascular.
Advances in hematology-oncology treatments have increased the number of survivors of childhood cancer and lengthened survival times. Nonetheless, people diagnosed with cancer during childhood are 7 times more likely to die of heart disease than the general population.25–29 This increased risk has multiple causes and depends a number of factors, described below.
- •
Type of cancer. Kidney cancers (eg, Wilms tumor), bone sarcoma, lymphoma (especially Hodgkin), and leukemia are associated with a higher CV risk because of the greater cardiotoxicity of their treatments and/or their association with CV risk factors.25,80
- •
Direct hematology-oncology treatment toxicity. Anthracyclines, alkylating agents, and radiotherapy are the most cardiotoxic treatments for pediatric patients.81–83 Over 50% of children with cancer are treated with anthracyclines, and the strongest predictor of cardiac dysfunction is total cumulative dose. Although there are no safe doses, total adriamycin doses should not exceed 450mg/m2 (or the equivalent for other anthracyclines). Toxic effects are exacerbated in patients treated with concomitant radiotherapy to the chest or high abdomen ().
- •
High prevalence of CV risk factors. Hematology-oncology treatments increase the vulnerability of the CV system and favor the development of CV risk factors and metabolic syndrome.82 The risk of kidney failure is also increased, particularly in very young children, children exposed to corticosteroids, and female patients. An association between CV risk factors and mortality after childhood cancer has been identified in numerous registries80,83–88 (). Adequate control of CV risk factors reduces the incidence of CV events; raising awareness of the importance of prevention from an early age is thus a priority.
A number of specific tools exist for assessing CV risk in children with cancer, such as the CV risk calculator from the Children's Cancer Survivors Study, which predicts the risk of heart failure, ischemic heart disease, and stroke by the age of 50 years. The tool calculates age at diagnosis, hematology-oncology treatments received (anthracyclines and maximum dose, alkylating agents, and platinum agents), concomitant radiotherapy (fields and doses), and presence of CV risk factors (diabetes, hypertension, dyslipidemia). Calculation of individual CV risk29,89–93 in patients transitioning to adulthood will identify those requiring closer follow-up by CHO units.
CV risk factor control goals must be met in survivors of childhood cancer. Targets are expressed in age or sex percentiles (p) or absolute values and include body mass index <p95, blood pressure <130/80mmHg or <p90, low-density lipoprotein cholesterol <130mg/dL (<100mg/dL in high-risk settings such as hematopoietic stem cell transplant), non–high-density lipoprotein cholesterol <145mg/dL, triglycerides <150mg/dL, fasting blood glucose <100mg/dL, and hemoglobin A1c <5.7%.82
Cancer and CV Risk: Pending IssuesA number of scientific issues regarding CV risk in patients with cancer remain to be resolved. Of particular note:
- •
Current CV risk models do not include a past or present history of cancer and associated treatment as modifiers of CV risk. No specific scales exist to assess this risk in patients with cancer.
- •
Cancer patients are underrepresented in CV prevention trials. Risk factor control goals are based on data from people without cancer who have the same CV risk. No randomized CV prevention trials have included patients with a history of cancer. Current recommendations are therefore based on expert consensus.
- •
Evidence is lacking on the usefulness of preventive therapies before initiation of hematology-oncology treatment.
- •
Robust evidence on the impact of CHO programs and CV rehabilitation on the prognosis of cancer survivors is needed.
- •
Evidence is also lacking on cost-effective long-term strategies for managing CV risk in cancer survivors.
- •
Coordinated multidisciplinary action is needed to facilitate the management and achievement of CV risk control goals in patients with cancer and to favor a risk-free return to social activities and work.
We have prepared a list of final recommendations based on the conclusions of this consensus statement to simplify and encourage their application in practice (table 6). Consensus was achieved for all recommendations using a rigorous Delphi method involving all the authors who have signed this consensus statement (). The details of the methodology used to obtain consensus are given in the corresponding section in (”).
Final recommendations
| Stratification of CV risk in patients with cancer: risk-modifying factors |
| • Patients with cancer are at least twice as likely as the general population to die of CV disease; periodic assessment and strict monitoring is therefore necessary throughout the different stages of cancer care. |
| • CV risk factor control is the responsibility of all health care professionals involved in cancer care, with support, where necessary, from cardiology. |
| • Stratification of CV risk helps establish control goals, initiate targeted prevention strategies, and plan follow-up programs adapted to CV risk. This risk assessment should be performed before the patient starts hematology-oncology treatment and periodically thereafter for the duration of care provision. |
| • No specific scales currently exist to assess CV risk in patients with cancer. Scales that have been validated in the general population, such as the SCORE (Systematic Coronary Risk Evaluation) chart, underestimate risk in this population. When assessing CV risk, it is necessary to consider specific modifying factors such as age at diagnosis, type of cancer and treatment, presence of metastatic disease, and history of thoracic radiotherapy (time of administration, dose, and technique), and/or hematopoietic stem cell transplant. |
| • Thoracic radiotherapy with inclusion of the heart in the radiation field progressively increases the risk of atherosclerotic CV disease; strict control of CV risk factors is thus necessary. |
| • In addition to stratifying for CV risk in patients with cancer, consider assessing comorbidities, frailty, and life expectancy. |
| • The increased incidence of CV disease in survivors of childhood cancer means that CV risk assessment and strict monitoring and control of CV risk factors are necessary from the moment of diagnosis. |
| CR risk factor treatment goals in patients with cancer: follow-up recommendations according to CV risk |
| • CV risk factor control goals should be established in accordance with current clinical guideline recommendations and estimated CV risk. |
| Start treating blood pressure in patients with a level >140/90mmHg with the goal of lowering it to ≤ 130/80mmHg, provided the treatment is well tolerated. |
| Maintain hemoglobin A1c values <7.0%; this target can be relaxed in older or frail patients and patients with a reduced life expectancy or comorbidities. |
| Maintain low-density lipoprotein cholesterol levels <116 mg/dL for patients with a low CV risk, <100 mg/dL for those with a moderate risk, <70mg/dL for those with a high risk, and <55mg/dL for those with a very high risk. |
| CV risk factor control goals can be relaxed in frail patients and patients with a reduced life expectancy due to their cancer and/or comorbidities. |
| • Follow-up of cancer survivors without CV symptoms and with a low CV risk should include an annual clinical evaluation and blood tests including lipid and glycemic profiles. |
| • Follow-up of cancer survivors without CV symptoms and with a moderate CV risk should include an annual check-up, an electrocardiogram, and blood tests including lipid and glycemic profiles. |
| • Follow-up of asymptomatic patients with a high risk of peripheral artery disease must include a targeted history, physical examination, and determination of ankle-brachial index. |
| Recommendations for reducing CV risk in patients with cancer |
| • CV risk factor control in patients with cancer should be approached using similar measures to those used in the general population, with lifestyle changes and/or pharmacological interventions. |
| • Patients with cancer should follow a Mediterranean diet, perform at least 150minutes of aerobic exercise combined with resistance training a week, and quit tobacco. |
| • Priority should be given to angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers. If vascular endothelial growth factor inhibitors are used, they should be combined with dihydropyridine calcium channel blockers. |
| • Statins are the first-line treatment for dyslipidemia. If contraindicated or if treatment needs to be intensified, ezetimibe and PCSK9 inhibitors are recommended. |
| • In patients with diabetes and cancer, antidiabetic drugs with a demonstrated benefit in reducing CV events should be prioritized. |
| • Primary care, occupational health, nursing-led patient education teams have a key role in assessing, monitoring, and controlling CV risk factors in patients with cancer. Patients must be informed of their CV risk and goals to ensure their engagement. |
CV, cardiovascular.
A. Martín García has received speakers’ fees from Daichii-Sankyo and done consultancy work for Bayer and Pfizer. In this and all the other cases listed below, there was no relationship to this study. C. Mitroi has received personal fees from Bayer and Janssen and nonfinancial support from Abbott. P. Mazón Ramos has received personal fees from Bayer, Boehringer-Lilly, AstraZeneca, Esteve, Novo-Nordisk, Mylan, and Daichii-Sankyo. R. García Sanz received funding from Hospital Universitario de Salamanca and Sociedad Española de Hematología during this study and grants and personal fees from Janssen-Cilag, Gilead, Takeda, Roche, BeyondSpring, and Novartis. F. Ayala de la Peña has received fees from AstraZeneca, Celgene, Eisai, Novartis, Roche, Pfizer, and Pierre Fabre; nonfinancial support from Roche, Pfizer, Celgene, Eisai, Pfizer, Pierre Fabre, and MSD; and grants from Celgene and Roche. J. Cosín-Sales has received speakers’ fees from Amgen, Sanofi, Almirall, Mylan, Astra-Zeneca, and Mundipharma and done consultancy work for Amgen, Sanofi, Almirall, Boehringer-Ingelheim, AstraZeneca, and Mundipharma. T. López Fernández has received speakers’ fees from Bayer, Philips, Pfizer/BMS, Daiichi-Sankyo, ROVI, Amgen, Janssen, and MSD. The other authors declare no conflicts of interest.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.11.020