The objective of this study was to determine the diagnostic yield of a stepped protocol involving an electrophysiologic study (EPS) and implantable loop recorders (ILR) in patients with syncope and bundle branch block (BBB).
MethodsEighty-five consecutive patients referred for syncope and BBB after initial non-diagnostic assessment underwent EPS including a pharmacological challenge with procainamide. Those patients without indication for defibrillator implantation received ILRs. Follow-up continued until diagnosis or end of battery life.
ResultsThe EPS was diagnostic in 36 patients (42%). The most frequent diagnoses were paroxysmal atrioventricular block (AVB) (n=27), followed by ventricular tachycardia (VT) (n=6). All patients with VT had structural heart disease; left BBB was more prevalent in this group. Thirty-eight patients received ILRs and diagnosis was achieved in 13 (34%) of them; paroxysmal AVB (n=10) was the most frequent diagnosis. Median follow-up to diagnosis of paroxysmal AVB was 97 days (interquartile range 60-117 days). Paroxysmal AVB was more frequent in patients with right BBB and prolonged PR interval and/or axis deviation. We found no occurrence of VT or arrhythmic death during follow-up.
ConclusionsThe most common etiology of syncope in patients with BBB was paroxysmal AVB, followed by VT. The stepped use of EPS and ILR in negative patients enables us to safely achieve a high diagnostic yield, given that VT is usually diagnosed during EPS.
Keywords
Syncope is a clinical condition responsible for a high rate of emergency room attention and a substantial number of admissions for diagnosis and treatment. Although most patients have a benign prognosis because the neurally mediated etiology is the most frequent, high rates of mortality in certain subgroups make it essential to obtain a precise diagnosis and apply adequate treatment.1
One particularly interesting subgroup consists of patients attending for syncope and presenting intraventricular conduction delay (IVCD) that compromises ≥1 His bundle fascicles. The presence of bundle branch block (BBB) in the 12-lead electrocardiogram (ECG) indicates paroxysmal atrioventricular block (AVB) as the underlying mechanism of syncope.2,3,4 Electrophysiologic study (EPS) with pharmacologic challenge and long-term monitoring with implantable loop recorders (ILR) are the two essential diagnostic tools in these patients when initial assessment fails to identify the cause of syncope.
Diagnosis and treatment in these patients with syncope and BBB was recently revised in European Cardiology Society clinical practice guidelines. The 2007 European guidelines on cardiac pacing and cardiac resynchronization therapy5 recommend cardiac pacing in patients with positive EPS (Class I, Level of Evidence C). In patients with normal His-ventricle (HV) interval, they consider pacemaker implantation an acceptable strategy as an alternative to loop recorder deployment (Class IIa, Level of Evidence C) and mention the prior exclusion of other causes of syncope, especially ventricular tachycardia. European guidelines on syncope, updated in 2009, recommend permanent cardiac pacing in patients with syncope and BBB following a positive EPS (Class IIa, Level of Evidence: B). They also mention that pacemaker implantation should be considered in patients with unexplained syncope and BBB (Class IIa, Level of Evidence C).6,7
Hence, current recommendations for patients without diagnostic EPS are mainly derived from expert panels and the high risk of progression to advanced AVB observed in small series.8 However, at the time of this writing, no large prospective series have established the safety of this approach. In the present study we describe the diagnostic yield and safety of a study protocol for syncope in a series of consecutive patients with IVCD.
MethodsWe included all patients referred to the arrhythmia unit of our center between January 2005 and June 2009 for invasive EPS, indicated for ≥1 episode of syncope or presyncope in the presence of IVCD and an initial clinical examination that did not yield a diagnosis.6,7
We defined IVCD as the presence of a QRS complex of ≥120ms duration. Left and right BBB were diagnosed using standard criteria.9 Anterosuperior hemiblock was defined as presence of a frontal axis ≤–45° with rS in DII and DIII; posteroinferior hemiblock was defined as the presence of a frontal axis ≥+100° with qR in DII and DIII, in the absence of right ventricular hypertrophy.
Study ProtocolInitial assessment consisted of clinical record review, physical examination, carotid sinus massage, 12-lead electrocardiogram (ECG), transthoracic Doppler echocardiography, and orthostatic testing. Other complementary tests (stress test, catheterization, electroencephalogram, cranial computerized tomography, etc.) were only used when, following initial examination, they were considered necessary to make a diagnosis. We excluded patients with narrow QRS (<120 ms) or clear diagnosis of the cause of syncope after initial assessment.
The EPS consisted of: a) baseline record of atrioventricular conduction times; b) massage of left and right carotid sinuses with continuous monitoring on ECG and continuous blood pressure monitoring; c) atrial pacing at increasing frequencies to determine the Wenckebach point of atrioventricular conduction and sinus node recovery time; d) atrial extra-stimulus test; e) ventricular pacing at increasing frequencies; f) ventricular extra-stimulus test in the apex and right ventricular outflow tract in two cycles, with ≤3 extra-stimuli; g) if the baseline HV interval was < 70ms and infrahisian AVB was not observed with atrial stimulation at increasing frequencies, we administered pharmacologic overload with 10mg/kg IV procainamide at 100mg/min. In those patients studied up to July 2008 we performed the ATP (adenosine triphosphate) test, administering a rapid 20mg IV bolus.
The EPS was considered to have diagnosed sinus dysfunction when it identified a corrected sinus recovery time of>550ms and paroxysmal AVB in the presence of: a) ≥70ms baseline HV interval or evidence of intrahisian or infrahisian block at baseline or with atrial stimulation at increasing frequencies, and b) either of the following after procainamide challenge: appearance of intrahisian or infrahisian block during spontaneous rhythm or after atrial or ventricular stimulation, or lengthening of the HV interval to>100ms or to a 100% increase over baseline. Diagnosis of supraventricular or ventricular tachycardia was attained when programmed stimulation induced any of these arrhythmias both reproducibly and consistently. In one patient, the EPS diagnosis was of paroxysmal AVB due to exaggerated atrioventricular node sensitivity to ATP, observed when presenting>9s ventricular pause following ATP administration.
After EPS, we implanted ILRs (REVEAL®, Medtronic Inc., Minneapolis, Minnesota, USA) in patients with no diagnosis and no indication for an implantable cardioverter-defibrillator (ICD) under current clinical practice guidelines. Ambulatory follow-up was at 3 months and at subsequent 6-month intervals, unless symptoms required an earlier visit. Diagnosis of the mechanism underlying syncope was established when recurrent syncope was recorded via the ILR. We defined paroxysmal AVB as second-degree type II or third-degree AVB when this was not preceded by progressive sinus bradycardia, given that this is a commonly observed response during neurally mediated reactions.
Statistical AnalysisCategorical data are presented as number and percentage. Continuous data are presented as mean ± standard deviation or median [25-75 percentiles] as appropriate. Normally distributed continuous variables were compared with Student's t test. For non-normal distributions, we used the Mann-Whitney U test. Categorical variables were compared with chi-square or Fisher exact test, as appropriate. A value of P<.05 was considered significant.
We constructed a multivariate logistic regression model to determine predictors of paroxysmal AVB. The dependent variable was diagnosis of paroxysmal AVB in EPS or by ILR in the follow-up. In the model, we included the covariables that were statistically significant in univariate analysis or of clinical interest. We constructed the model using stepwise 0.05 entry and 0.1 exit probabilities. We used the Hosmer-Lemeshow test to determine goodness-of-fit. To determine the model's discrimination capacity, we estimated the area under the ROC curve, 95% confidence interval (CI) and P (χ2). Analysis was with SPSS 16.0. 2004. (SPSS, Inc., Chicago, Illinois, USA).
ObjectivesThe pre-established primary objective of this study was to determine the diagnostic value of EPS and ILR in the population enrolled. The secondary objectives were to describe characteristics of patients diagnosed with paroxysmal AVB in EPS and by ILR, time to diagnosis, syncopal recurrence until diagnosis, baseline characteristics of IVCD subgroups and their clinical course.
ResultsBetween January 2005 and June 2009, 85 patients who fulfilled our inclusion criteria were referred to the electrophysiology laboratory. Baseline patient characteristics are in Table 1. Mean age was 72.6±9.8 years; 71.8% were men.
Table 1. Baseline Characteristics of Population *
| Age, years ± SD | 72.6±9.8 |
| Men | 61 (71.8) |
| Diabetes | 25 (29.4) |
| Dyslipidemia | 28 (32.9) |
| High blood pressure | 56 (65.9) |
| Smokers | 8 (9.4) |
| AFi/AFl | 18 (21.2) |
| Heart disease | 56 (67.5) |
| Hypertensive | 28 (33.7) |
| Ischemic heart disease | 19 (22.9) |
| Previous infarction | 11 (13.3) |
| Dilated | 5 (6) |
| Hypertrophic | 6 (7.2) |
| Moderate/severe valvular heart disease | 7 (8.4) |
| LVEF ≥45% | 69 (80.7) |
| LVEF<45% | 16 (19.3) |
| RBBB, without hemiblock | 16 (18.8) |
| LBBB | 31 (36.5) |
| RBBB+ASH | 30 (35.3) |
| RBBB+IPH | 4 (4.7) |
| Non-specific T | 4 (4.7) |
| Presyncope | 4 (4.7) |
| Single syncope | 29 (34.1) |
| ≥2 syncopes | 52 (61.2) |
| QRS, ms | 148.9 (18.3) |
| PR, ms | 193.9 (53.8) |
AFi, atrial fibrillation; AFl, atrial flutter; ASH, anterosuperior hemiblock; IPH, inferoposterior hemiblock; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; RBBB, right bundle branch block.
* Values are expressed mean±standard deviation or n(%).
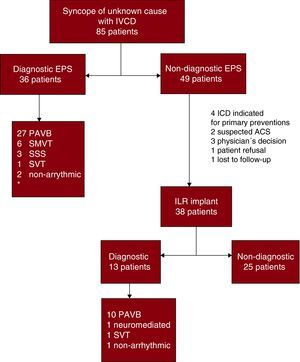
In 36 patients (42.3%) diagnosis was achieved by EPS (Figure 1). Paroxysmal AVB was the most frequent diagnosis in EPS, observed in 27 patients (31.7%), followed by sustained monomorphic ventricular tachycardia (SMVT) in 6 (7%). Other causes detected were: sinus dysfunction in 3 patients (3.5%), supraventricular tachycardia in 1 (1.1%) and pseudosyncope of vascular origin in 1 (1.1%). In 1 patient (1.1%) referred for syncope with initial cardiac arrhythmia in QRS, the exclusion of ventricular pre-stimulation led to a diagnosis of non-arrhythmic syncope.
Figure 1. Electrophysiologic study and loop recorder results. Flow chart. ACS, acute coronary syndrome; EPS, electrophysiologic study; ICD, implantable cardioverter-defibrillator; IVCD, intraventricular conduction delay; ILR, implantable loop recorder; PAVB, paroxysmal atrioventricular block; SMVT, sustained monomorphic ventricular tachycardia; SSS, sick sinus syndrome; SVT, supraventricular tachycardia. *The table reports numbers of diagnoses and not numbers of patients because some patients had more than one diagnosis.
Three (8.3%) of the patients diagnosed by EPS met diagnostic criteria for ≥1 etiology: 2 for paroxysmal AVB and SMVT and 1 for paroxysmal AVB and sinus dysfunction.
Implantable Loop RecordersIn 49 (57.6%) of the 85 patients studied, the EPS failed to achieve a diagnosis of the underlying etiology. Of these, 38 received an ILR (44.7%). In 11 of the initial 49 negative EPS patients, the loop recorder was not implanted, for the following reasons: patients with severe ventricular dysfunction were candidates for an ICD (n=4); clinical suspicion existed that acute coronary syndrome was the cause of signs and symptoms of syncope (n=2); decision of the attending physician (n=3); refusal on the part of the patient (n=1); lost to follow-up (n=1). We attained a diagnosis in 13 patients (34.2% of ILR receivers). The most common diagnosis was paroxysmal AVB, documented in 10 patients. Other causes were: neuromediated syncope (n=1), supraventricular tachycardia (n=1) and non-arrhythmic syncope (n=1).
Paroxysmal Atrioventricular BlockThe most frequent etiology diagnosed was paroxysmal AVB, found in 37 patients (43.5%). Of these, 27 (73%) were diagnosed by EPS and 10 (27%) by ILR. Time to diagnosis by ILR was 25-152 days, median 97 days [interquartile range, 60-117], and 4 patients presented syncopal recurrences prior to diagnosis. Median follow-up in patients without diagnosis by ILR was 449 [123-641] days; minimum 52, maximum 902 days.
In univariate analysis (Table 2), we found a significantly greater proportion of patients with diabetes mellitus, dyslipidemia, right BBB and first-degree AVB among those with final diagnosis of paroxysmal AVB (whether by EPS or ILR). We excluded 11 patients from multivariate analysis because they did not complete the diagnostic assessment (Figure 1). We identified diabetes mellitus, right BBB and prolonged PR interval as independent predictors of paroxysmal AVB (Table 3). The Hosmer-Lemeshow goodness-of-fit test showed no evidence of poor fit of the model proposed (χ2=9.344; P=.314). The area beneath the ROC curve of the final model to predict paroxysmal AVB was 0.81 (95% CI, 0.71-0.91; P<.001).
Table 2. Variables Associated With Paroxysmal Atrioventricular Block. Univariate Analysis.
| PAVB (37 patients) | No PAVB (37 patients) | P | |
| Age (years) | 73±7 | 71±12 | NS |
| Men | 30 (81) | 27 (73) | NS |
| Diabetes | 17 (46) | 5 (13) | .002 |
| High blood pressure | 22 (59) | 27 (73) | NS |
| Dyslipedemia | 18 (48) | 8 (21) | .01 |
| Heart disease | 22 (61) | 25 (69) | NS |
| LVEF<45% | 5 (14) | 6 (17) | NS |
| RBBB | 30 (81) | 18 (51) | .009 |
| LBBB | 7 (19) | 17 (46) | |
| PR (ms) | 218±61 | 176±39 | .001 |
| QRS (ms) | 150±19 | 147±16 | NS |
| ≥2 syncopes | 27 (73) | 21 (57) | NS |
LBBB, left bundle branch block; LVEF, left ventricular ejection franction; PAVB paroxysmal atrioventricular block; RBBB, right bundle branch block. Values are expressed as mean±standard deviation or n (%).
Table 3. Results of Multivariate Analysis for Diagnosis of Paroxysmal Atrioventricular Block.
| OR | 95% CI | P | |
| RBBB | 3.80 | 1.12-12.8 | .03 |
| PR (per 10ms PR change) | 1.19 | 1.03-1.37 | .01 |
| Diabetes | 3.89 | 1.06-14.2 | .03 |
CI, confidence interval; OR, odds ratio; RBBB, right bundle branch block.
Analysis of subgroups of patients with different forms of IVCD or absence of first degree AVB showed more patients diagnosed with paroxysmal AVB among those with right BBB associated with anterosuperior hemiblock and/or first-degree AVB. Fewer diagnoses of paroxysmal AVB were found among patients with non-specific IVCD or left BBB not associated with pathologically prolonged PR interval. Table 4 shows data for each form of IVCD.
Table 4. Diagnosis of Paroxysmal Atrioventricular Block According to Type of Conduction Disturbance.
| N | Diagnosis of PAVB | Diagnosis by EPS | Diagnosis by ILR | |
| RBBB+ASH+AVB-1 | 18 | 13 (72.2%) | 11 | 2 |
| RBBB+AVB-1 | 4 | 3 (75%) | 3 | 0 |
| RBBB+ASH | 12 | 7 (58.3%) | 5 | 2 |
| Isolated RBBB | 12 | 5 (41.6%) | 2 | 3 |
| RBBB+IPH | 4 | 2 (50%) | 0 | 2 |
| LBBB+AVB-1 | 8 | 3 (37.5) | 3 | 0 |
| Isolated LBBB | 23 | 4 (17.3%) | 3 | 1 |
| Non-specific IVCD | 4 | 0 (0%) | 0 | 0 |
| Total | 85 | 37 (43.5%) | 27 | 10 |
ASH, anterosuperior hemiblock; AVB-1, first-degree atrioventricular block; EPS, electrophysiologic study; IPH, inferoposterior hemiblock; ILR, implantable loop recorders; IVCD, intraventricular conduction delay; LBBB, left branch bundle block; PAVB, paroxysmal atrioventricular block; RBBB, right branch bundle block. Values are expressed as n (%).
In our study, a final diagnosis of SMVT was achieved in only 6 patients (7%). All presented structural heart disease (5 with ischemic heart disease and 1 with hypertrophic cardiomyopathy) and only 1 patient had>45% LVEF. Presence of left BBB was significantly greater in patients with SMVT than in those without SMVT (66% and 36%, respectively; P=.03). All diagnoses of SMVT were attained by EPS and we observed no episodes of ventricular tachycardia in the ILR follow-up or deaths attributable to VT.
We recorded 2 deaths (2.3%) during follow-up, neither of which was caused by an arrhythmia. One patient lost to follow-up after a non-diagnostic EPS died of a coronary cause and another died of leukemia during follow-up and while carrying an ILR.
DiscussionThe principle finding of our study is the high prevalence of arrhythmic syncope (57%) in our population, represented in the main by paroxysmal AVB and IVCD, and the appropriateness of the sequence of diagnostic tests used, enabling us to attain a high rate of diagnosis without increasing the level of risk for patients.
The existence of IVCD in patients with syncope raises the suspicion of paroxysmal AVB as the cause.10,11,12 The EPS and prolonged ECG monitoring, with ILR if necessary, are the diagnostic tools available.
If the contralateral branch of the His-Purkinje system is affected in patients with BBB, it is considered directly related with progress to high-degree AVB.13 This can appear in EPS when HV interval is measured, at baseline or after overloads, or in programmed stimulation or administration of sodium channel blockers.14 However, as the ISSUE study reported, a large proportion of patients with syncope, BBB and non-diagnostic EPS subsequently require permanent cardiac pacing, whether for AVB or sinus node dysfunction.2
The frequent need for cardiac pacing in midterm follow-up justifies the trend observed in clinical practice guidelines to consider pacemaker implantation despite non-diagnostic EPS. Some authors have argued for immediate pacemaker implantation, without prior invasive EPS.8 However, to date, no large prospective series exist that prove the safety of this approach.
An alternative strategy, aimed at diagnosing patients requiring permanent cardiac pacing and avoiding unnecessary implants, is the use of ILR.
Some time ago, the anatomic characteristics of the trifascicular His-Purkinje system were described, isolating the anterosuperior part of the left branch as thinner and more fragile, and the posteroinferior part as the more vulnerable. In fact, according to Elizari et al, the presence of right BBB associated with posteroinferior hemiblock should be considered a precursor to complete AVB.15 However, the risk of progression to high-degree AVB following syncope in the different forms of IVCD is not fully understood and the clinical practice guidelines fail to advise as to whether it should be treated differently.5,6,7,16 In our patients, multivariate analysis detected right BBB with prolonged PR interval as the marker most frequently associated with diagnosis of paroxysmal AVB both by EPS and ILR. This contrasts with the lack of predictive patterns reported by Tabrizi et al,8 although they only considered bifascicular block and did not analyze PR interval. In this respect, ISSUE2 was similar, only including patients with negative EPS and ILR follow-up and identifying baseline characteristics associated with development of paroxysmal AVB. The ISSUE authors did, however, observe a “protective” tendency for right BBB without axis deviation or for>2-year history of syncopal episodes. In our series, the only clinical characteristic significantly associated with diagnosis of paroxysmal AVB was diabetes mellitus, which may be expressed by a population with greater comorbidity and, therefore, with greater fibrous effect on the conduction system. In our series, the low number of patients with IVCD, traditionally associated with greater risk of high-degree AVB such as right BBB with posteroinferior hemiblock, may explain why they are under-represented in the multivariate model. In a multivariate model, the search for factors that predict AVB could teach us about clinical variables (essentially comorbidity) or variables derived from diagnostic tests (essentially electrocardiographic) that are associated with greater risk, and thereby simplify therapeutic decision-making. The number of patients included in our series prevents us from exploring these possibilities.
It has been observed that in this subgroup of patients another important and serious cause of syncope is ventricular tachycardia, particularly in patients with deteriorated left ventricular systolic function.17 In our study, they constituted 7% of cases. Left BBB was the IVCD most frequently associated with SMVT diagnosis, probably associated with the structural heart disease.
The not inconsiderable prevalence of ventricular arrhythmia justifies a strategy like that followed in our study, in which direct pacemaker implantation did not take place, given that it would have meant implanting patients who did not need it and, above all, ignoring much more dangerous diagnoses such as SMVT. In fact, in the series of 27 patients with bifascicular BBB and syncope reported by Tabrizi et al,8 in which they proceeded to direct pacemaker implantation (without previous EPS), a 19% rate of sudden death was observed during the follow-up. The EPS and subsequent ILR implantation in negative cases has proved, in its turn, a beneficial, safe strategy in patients with suspected arrhythmic syncope.18,19
The EPS produced a diagnosis in 45.8% (36 patients); 75% were of paroxysmal AVB and 16.6% of SMVT. Among patients with negative EPS and an ILR, 26% (38) had paroxysmal AVB in the follow-up, a figure similar to that reported elsewhere.2 Note that all diagnoses of SMVT (6 patients; 7%) were achieved by EPS in patients with heart disease and left ventricular systolic dysfunction, although 3 did not meet primary prevention criteria for defibrillator implantation. This, together with the fact that we observed no arrhythmic mortality during the follow-up of ILR patients, confirms the safety of the strategy used and demonstrates that, despite less-than-optimal diagnostic sensitivity, the value of EPS is that it excludes from follow-up those patients at greater risk.
In fact, in a hypothetical alternative scenario considering presence of syncope with bifascicular BBB as adequate indication for pacemaker implantation, in our series we would find that after defibrillator implant in 7 patients meeting primary prevention criteria, 59 of the remaining 78 patients would have received a pacemaker, including the 3 patients in whom ventricular tachycardia was induced in the EPS and excluding 8 patients without bifascicular BBB in whom paroxysmal AVB was demonstrated in the ILR follow-up.
Finally, the high prevalence of arrhythmic syncope (57%) and high-degree AVB (43.5%) found in our study contrasts with the lower prevalence reported in other studies. Recently, Romero et al20 reported on a specialized syncope clinic in which 82 patients discharged from ER with ≥2 OESIL scale risk criteria (age>65 years, syncope without previous signs or symptoms, abnormal baseline ECG, known cardiovascular disease). After a mean 2.1 year follow-up, only 12% had a final diagnosis of arrhythmic syncope. This finding that conflicts with the present study could be due to differences in the population included, as selection was more restrictive in our series. Romero et al report that fewer than half of their patients presented IVCD and the diagnostic procedures conducted were mainly those used in our “initial assessment”. The use of invasive procedures was minimal (4 EPS and 2 ILR) and the most frequent diagnoses were neuromediated and orthostatic syncope. In contrast, in our series presence of IVCD was an inclusion criterion and we systematically excluded patients with a clear diagnosis following initial assessment.
LimitationsOur study was conducted in a series selected from a single center, enrolled in an arrhythmia unit specifically dedicated to the study of syncope, which could imply a degree of bias. However, the principle bias likely to arise is the over-representation of arrhythmic causes of syncope, which should not constitute a problem, given that we specifically sought to analyze the search for arrhythmic syncope.
One limitation of our study is that 11 patients with negative EPS did not complete the diagnostic schema because they did not receive ILR for various reasons. In more than half of these cases, implantation did not occur because of primary prevention indication for defibrillator implantation or because a prior alternative diagnosis made continuation of the diagnostic process unnecessary. As these patients did not take part in the ILR follow-up, we may have reduced the number of diagnoses attained by ILR, which would underestimate its diagnostic capacity.
Due to sample size, we could not determine with precision whether presence of autoimmune hepatitis in addition to right BBB entails greater risk of paroxysmal AVB. It would be interesting to explore this hypothesis in future research. Moreover, probably due to the small sample size, the multiple logistic regression Odds Ratio 95% CI ranges are broad and, therefore, imprecise.
Finally, as in other studies, abnormal EPS or ILR results were considered equivalent to a diagnosis of the real cause of syncope. Although the diagnostic criteria used in the present study are broadly accepted, in the future their meaning should be clarified in specific subgroups of patients through the results of clinical trials with a larger number of patients and longer follow-up.
ConclusionsIn patients with IVCD, paroxysmal AVB is the most frequent cause of syncope and the most relevant markers are right BBB with prolonged PR interval and diabetes mellitus. Ventricular tachycardia is the next most frequent cause of syncope in these patients, being left BBB most frequent in these cases. The stepped use of EPS followed by ILRs in negative patients enables us to make a high percentage of diagnoses with certainty, given that ventricular tachycardia is usually identified in the EPS.
Conflicts of interestNone declared.
Acknowledgements
The authors extend their sincere thanks to Dr Mariana Galante for her advice and collaboration in the statistical analysis.
Received 30 June 2010
Accepted 17 October 2010
Corresponding author: Unidad de Arritmias, Servicio de Cardiología, Hospital Clínico Universitario, Avda. Blasco Ibáñez 17, 46010, Valencia, Spain. damiazo@gmail.com