Postinfarction ventricular septal rupture is a rare but severe complication of myocardial infarction with high mortality rates. Our goal was to analyze which factors could have an impact on mortality due to this entity over the past decade, including those related to mechanical circulatory support.
MethodsThe CIVIAM registry is an observational, retrospective, multicenter study carried out in Spain. We designed a comparative analysis, focused on description of in-hospital management and in-hospital and 1-year total mortality as the primary endpoints, dividing the total observation time into 2 equal temporal periods (January 2008 to June2013 and July 2013 to December 2018).
ResultsWe included 120 consecutive patients. Total mortality during this period was 61.7% at 1-year follow-up. Patients in the second period were younger. One-year mortality was significantly reduced in the second period (75.6% vs 52.7%, P=.01), and this result was confirmed after adjustment by confounding factors (OR, 0.40; 95%CI, 0.17-0.98). Surgical repair was attempted in 58.7% vs 70.3%, (P=.194), and percutaneous closure in 8.7% and 6.8%, respectively (P=.476). Heart transplant was performed in 1 vs 5 patients (2.2% vs 6.8%, P=.405). The main difference in the clinical management between the 2 periods was the greater use of venoarterial extracorporeal membrane oxygenatiom in the second half of the study period (4.4% vs 27%; P=.001).
ConclusionsPostinfarction ventricular septal rupture still carries a very high mortality risk. There has been a progressive trend to increased support with venoarterial extracorporeal membrane oxygenatiom and greater access to available corrective treatments, with higher survival rates.
Keywords
In 1875, Latham et al.1 first described ischemic ventricular septal rupture (VSR) and since then it has been considered one of the most serious complications of acute myocardial infarction (AMI). Currently, the epidemiology of VSR has changed with a prevalence of 0.2% to 0.3%, in comparison with the estimated 3% rates before widespread reperfusion therapies.2–4 However, survival in affected patients does not appear to have improved. Contemporary registries show extraordinarily high 30-day mortality rates, between 38% and 88%, due to the development of cardiogenic shock and multiorgan failure.3,4 Widespread application of stent-based reperfusion strategies has probably helped to reduce the rates of AMI-related mechanical complications, but despite continuous technical improvements in interventional cardiology, in-hospital mortality does not appear to be decreasing.5–7
The early adoption of mechanical circulatory support could represent a turning point in the management of mechanical complications, such as VSR or papillary muscle rupture. The hemodynamic improvement can lead to adequate stabilization, avoiding the systemic consequences of low tissue perfusion and multiorgan failure before corrective surgery.8–14 This strategy has shown positive results even in patients with a high-risk profile assessed by the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) classification and with established multiorgan failure.14 Nonetheless, although early identification and support of cardiogenic shock seems to change the poor prognosis of post-AMI VSR, the evidence remains limited. Moreover, current data on VSR in the era of optimal reperfusion with primary percutaneous coronary intervention (PCI) is scarce.
In this study, we aimed to analyze developments in the management and prognosis of post-AMI VSR patients in a multicenter registry involving tertiary centers with well-developed reperfusion networks and locally available or rapid access to cardiac surgery facilities.
METHODSStudy design, population, and data collectionThis observational, retrospective and multicenter compared differences in the treatment and prognosis of patients with post-AMI VSR during the last decade.
After the study was approved by institutional review boards, we selected all consecutive patients with post-AMI VSR between January 1, 2008, and December 31, 2018 from each local database. An invitation was sent to 11 tertiary hospitals in Spain, located in different geographical regions and with available organized reperfusion networks. Each institution has either on-site cardiac surgery or easy access to rapid transfer of patients with mechanical complications and access to electronic medical history, where data of the event and follow-up was obtained. There were no exclusion criteria. Definitive diagnosis of VSR was obtained by Doppler echocardiography or cardiac catheterization. The overall availability of AMI networks for early reperfusion in different regions from included centers is available in table 1 of the supplementary data. The database was created with the information available from the electronic registries and specific individual databases of the cardiovascular intensive care unit.
Clinical endpointsThe primary endpoint was all-cause mortality at 1-year of follow-up. We analyzed in-hospital mortality as a co-primary endpoint. We specifically compared outcomes between the first half of the recruitment period and the second half. Secondary endpoints were temporal trend changes in the treatment, including revascularization, mechanical circulatory support, such as venoarterial extracorporeal membrane oxygenatiom (VA-ECMO) and intra-aortic balloon pump, used in isolation or combined, and VSR surgical or percutaneous closure.
Statistical analysisFor time-trend analysis, patients were divided in 2 periods, January 1, 2008 to June 30, 2013, and from July 1, 2013 to December 31, 2018.
Patient characteristics were summarized with continuous variables expressed as means (standard deviation), or median [interquartile range] if the distribution was nonnormal, and categorical variables are expressed as frequencies and percentages.
For the first step, we performed a univariate analysis. Characteristics were compared across both groups using the t test for normally distributed continuous variables and the Wilcoxon test for those with a skewed distribution. Categorical dichotomous variables were compared using the chi-square t test or the Fisher exact test when appropriate. Categorical nondichotomous variables were compared using ANOVA test.
For the second step, we performed a multivariate analysis with Cox regression. In the multivariate analysis model, we included all statistically significant variables identified in the univariate analysis. Additionally, other preselected variables influencing prognosis were also included: age, diabetes, AMI revascularization, successful VSR repair, and VA-ECMO use. Confounding factors were assessed by the change-in-estimate method using a threshold of 10% of variation in adjusted hazard ratios (aHR).15,16
The change-in-estimate method for analyzing confounding factors compares aHR between the models, with all previously selected variables and all the possible models, creating a different combination of these selected variables. If aHR deviated more than 10% from the maximum model aHR, confounding bias was present, and the variable was to be included in the final model. After the regression model, variance inflation factor was used to exclude multicollinearity.
Both models were built for in-hospital mortality and 1-year mortality. The final model was expressed by aHR (95% confidence interval [95%CI]). Due to the heterogeneous origin of the patient data, a deviance analysis was subsequently performed to confirm the equi-dispersion in the sample.
The estimation of the incidence of VSR in the study period was the result of the overall number of VSR divided by the extrapolated medium of cases reported in each hospital with available data. Information on survival status and 1-year follow-up was available for all patients.
All statistical analyses were performed using Stata/IC 15.1 for Windows (StataCorp LLC, United States).
RESULTSBaseline characteristics and incidence of ventricular septal ruptureA total of 120 patients fulfilled the inclusion criteria, 46 in the first period and 74 in the second period. The estimated incidence of VSR during the study period varied among centers, ranging 0.27% to 0.46% of the full AMI spectrum.
Baseline characteristics are listed in table 1. There were no significant differences between classic cardiovascular risk factors. Patients in the second period were younger. There were no significant differences in previous cardiovascular history between the 2 periods, including previous AMI, cerebrovascular disease, and peripheral arterial disease. The study population had high values of both the Charlson Comorbidity Index and EuroSCORE II, as additional predictors of poor prognosis, without significant differences between periods.
Baseline characteristics
| Total (n=120) | First period (n=46) | Second period (n=74) | P | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, y | 71.1±12.5 | 74.9±9.8 | 68.8±13.4 | .009 |
| Female sex | 51 (42.5) | 24 (52.2) | 27 (36.5) | .091 |
| Hypertension | 69 (57.5) | 31 (67.4) | 38 (51.4) | .309 |
| Diabetes | 40 (33.3) | 19 (41.3) | 21 (28.4) | .144 |
| Dyslipidemia | 52 (43.3) | 15 (32.6) | 37 (50) | .062 |
| Smoking | ||||
| Never | 70 (58.3) | 28 (60.9) | 42 (56.8) | .102 |
| Current | 41 (34.2) | 12 (26.1) | 29 (39.2) | .102 |
| Former | 9 (7.5) | 6 (13.0) | 3 (4.1) | .102 |
| BMI, kg/cm2 | 26.8±4.0 | 26.9±3.6 | 26.7±4.2 | .795 |
| Previous cardiovascular disease | ||||
| Previous NSTEMI | 4 (3.3) | 2 (4.4) | 2 (2.7) | .637 |
| Previous STEMI | 5 (3.3) | 2 (4.4) | 2 (2.7) | .637 |
| Previous PCI | 6 (5) | 3 (6.5) | 3 (4.1) | .674 |
| Previous CABG | 2 (1.6) | 0 (0) | 2 (2.7) | .523 |
| Peripheral arterial disease | 8 (6.6) | 2 (4.4) | 6 (8.1) | .709 |
| Cerebrovascular disease | 4 (3.3) | 2 (4.4) | 2 (2.7) | .633 |
| Prognostic scores | ||||
| EuroSCORE II | 19.8±16.2 | 18.6±13.6 | 20.5±17.6 | .535 |
| Charlson score | 4.8±2.4 | 4.5±2.1 | 4.9±2.5 | .330 |
BMI, body mass index; CABG, coronary artery bypass grafting; NSTEMI, non–ST-elevation acute myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
The data are expressed as No. (%) or mean±standard deviation.
The main characteristics of the ischemic episode are summarized in table 2. The prevalence of anterior and inferior ST-segment elevation myocardial infarction was similar in the 2 periods, as well as the distribution between culprit lesions on left anterior descending artery or right coronary artery. Apical location of VSR was more frequent than basal, with no significant differences between groups. The estimated size of the defect was larger in the second study period, but this datum was only available for 71 patients.
Characteristics of the ischemic event
| Total (n=120) | First period (n=46) | Second period (n=74) | P | |
|---|---|---|---|---|
| Myocardial infarction features | ||||
| ST elevation | .606 | |||
| Anterior | 42 (35) | 16 (45.5) | 26 (37.8) | |
| Inferior | 50 (41.6) | 15 (34.1) | 35 (47.3) | |
| Non–ST elevation | 1 (0.1) | 0 (0) | 1 (1.4) | |
| Coronary angiography | 99 (82.5) | 34 (75.6) | 65 (87.8) | .082 |
| Culprit lesion | .394 | |||
| No significant | 3 (2.5) | 1 (2.9) | 2 (3.1) | |
| Stenoses LMCA | 2 (1.6) | 0 (0) | 2 (3.1) | |
| LAD | 41 (34.2) | 16 (47.1) | 25 (39.1) | |
| CX | 3 (2.5) | 2 (5.9) | 1 (1.6) | |
| RCA | 48 (40) | 14 (41.2) | 34 (53.1) | |
| Echocardiographic findings | ||||
| LVEF, % | 44.1±11.2 | 42.5±10.7 | 45.1±11.5 | .227 |
| Basal VSR | 41 (34.2) | 15 (32.6) | 26 (35.1) | .792 |
| Apical VSR | 71 (65.5) | 31 (67.3) | 48 (64.9) | .744 |
| VSR size, cm2 | 1.6±0.9 | 1.1±0.6 | 1.8±0.9 | .003 |
| Diagnosis and treatment delay | ||||
| STEMI diagnosis (d after initial symptoms)* | 2.4±5.2 | 2.1±5.0 | 2.7±5.2 | .557 |
| 1 [0-2] | 0 [0-2] | 0 [0-3] | ||
| Time from STEMI diagnosis to VSR diagnosis,*d | 2.3±7.3 | 2 [0-4.5] | 2 [0-6.5] | .694 |
| 0 [0-1] | 0 [0-1] | 0 [0-1] | ||
| Time from VSR diagnosis to attempted closure,*d | 2.6±3.5 | 3.3±4.9 | 2.1±2.4 | .586 |
| 1 [0-4] | 1.5 [0-4] | 1 [0-5] | ||
CX, circumflex artery; LAD, left anterior descending artery; LMCA, left main coronary artery; LVEF, left ventricular ejection fraction; RCA, right coronary artery; STEMI, ST-segment elevation myocardial infarction; VSR, ventricular septal rupture.
The data are expressed as mean±standard deviation and median [interquartile range].
Patients in this series frequently presented beyond an acceptable primary reperfusion time window, with the mean delay between initial symptoms and definitive diagnosis of the VSR being 2.3 ± 7.3 days in both groups (table 2). Of the 65 patients (55.6%) who presented in the first 24hours symptoms, only 31 (47%) received PCI. None of these patients received thrombolytic therapy before catheterization.
Post-AMI-VSR was diagnosed after a mean of 2.3±7.3 days after definitive diagnosis of AMI. The complication was detected after the first 24hours of admission in 19 patients in the first period and in 21 in the second period (41.3% vs 28.4%, P=.144).
There were no differences in coronary angiography and PCI rates, performed in the whole cohort in 99 patients (82.5%) and 52 patients (43.3%), respectively.
Three patients in our registry showed no significant epicardial artery disease on invasive coronary angiography (myocardial infarction with nonobstructive coronary atherosclerosis [MINOCA]). All patients were women with a median age of 64 years. None of these patients received thrombolytic therapy before catheterization. Two of these 3 patients died during hospitalization.
Management characteristicsManagement characteristics are summarized in table 3. There was a significant difference in the use of VA-ECMO between the 2 periods (4.4% vs 27%; P=.001), but there were no clear differences in the use of intra-aortic balloon pump (62.2% vs 71.2%; P=.309) or other mechanical circulatory support devices (8.7% vs 1.4%, P=.071). The number of days from VSR diagnosis to surgical repair was higher in patients with VA-ECMO, although this difference was not significant (2 [1-6] vs 5 [1-6] days; P=.2199, Wilcoxon test).
Management and outcomes
| Total(n=120) | First period(n=46) | Second period(n=74) | P | |
|---|---|---|---|---|
| IABP | 90 (75) | 28 (62.2) | 52 (71.2) | .309 |
| VA-ECMO | 22 (18.3) | 2 (4.4) | 20 (27.0) | .001 |
| Other MCS | 5 (4.1) | 4 (8.7) | 1 (1.4) | .071 |
| Percutaneous closure | 9 (7.5) | 4 (8.7) | 5 (6.76) | .476 |
| Surgical repair | 79 (65.8) | 27 (58.7) | 52 (70.3) | .194 |
| Combined VSR repair plus CABG | 20 (16.6) | 7 (15.2) | 13 (17.6) | .958 |
| CABG revascularization | 32 (26.6) | 11 (23.9) | 21 (28.8) | .561 |
| PCI revascularization | 52 (43.3) | 21 (46.7) | 31 (42.5) | .655 |
| Cardiac transplant | 6 (5) | 1 (2.2) | 5 (6.8) | .405 |
| Residual shunt* | 31 (25.8) | 9 (19.6) | 22 (29.7) | .746 |
| In-hospital mortality | 72 (60) | 33 (71.7) | 39 (52.7) | .038 |
| 1-year mortality | 74 (61.6) | 35 (75.6) | 39 (52.7) | .010 |
CABG, coronary artery bypass grafting; IABP, intra-aortic balloon pump; MCS, mechanical circulatory support; PCI, percutaneous coronary intervention; VA-ECMO, venoarterial extracorporeal membrane oxygenation; VSR, ventricular septal rupture.
The data are expressed as No. (%).
Percutaneous repair of the VSR was performed in a few patients during the study period (4 and 5 patients from the first and second periods, respectively, P=.476), and was associated with poor overall results in our study, with in-hospital mortality occurring in all patients, regardless of the initial success of device implantation.
Surgical repair was performed in 27 patients in the first period and 52 in the second period, with no significant differences (58.7% vs 70.3%; P=.194). Coronary artery bypass grafting was performed in addition to closure of the defect in 16.6% of the patients, with similar rates in both periods (15.2 vs 17.6%, P=.958). Despite attempted closure, some degree of residual or recurrent shunt occurred in 31 patients (25.8%), with a similar distribution between groups (table 3).
Regarding optimal timing of surgery in patients with VA-ECMO, patients who underwent surgical repair from day 4 had a significantly lower mortality rate than patients operated on in less than 24hours or those with a delay of 1 to 3 days (OR, 0.34; 95%CI, 0.12-0.94) (table 4).
Heart transplant was performed in 1 vs 5 cases, respectively (P=.405). Only 1 patient receiving a transplant died during hospitalization.
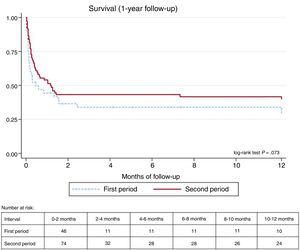
EndpointsIn-hospital and 1-year mortality was 60% and 61.7%, respectively. The results of the univariate analysis showed a statistically significant reduction in mortality in the second study period, including both in-hospital and 1-year mortality (table 3). Kaplan Meyers curves where created of survival rates in both groups during the first 12 months and are reflected in figure 1.
Regarding optimal timing of surgery in patients with VA-ECMO, patients who underwent surgical repair from day 4 had a significantly lower mortality rate than patients operated on in less than 24hours or those with a delay of 1 to 3 days (HR, 0.34; 95%CI, 0.12-0.94) (table 4).
Multivariate analysis included age, VA-ECMO use, diabetes, successful VSR repair and any revascularization therapy, throughout the study periods. The final models after Cox regression are shown in table 5. Older age was an independent predictor of both in-hospital and 1-year mortality. Management of VSR during the second half of the study was independently correlated with higher 1-year survival (HR, 0.40; 95%CI, 0.17-0.98; P=.045). A complete analysis of in-hospital mortality is summarized in table 2 of the supplementary data.
Results of the multivariate analysis
| Hazard ratio | 95%CI | P | |
|---|---|---|---|
| In-hospital mortality | |||
| Second period | 0.54 | 0.23-1.28 | .162 |
| Age | 1.06 | 1.02-1.11 | .004 |
| VA-ECMO | 1.98 | 0.64-6.11 | .234 |
| Diabetes | 1.38 | 0.59- 3.26 | .458 |
| 1-year mortality | |||
| Second period | 0.40 | 0.17-0.98 | .045 |
| Age | 1.06 | 1.02-1.11 | .008 |
| VA-ECMO | 1.87 | 0.60-5.76 | .278 |
| Myocardial revascularization | 0.77 | 0.33-1.80 | .544 |
95%CI, 95% confidence interval; VA-ECMO, venoarterial extracorporeal membrane oxygenation.
The main results of our study were the following: a) VSR is still a relatively rare complication of AMI with a high mortality rate; b) VSR patients have been more frequently managed with VA-ECMO support in the last few years, with a trend to more frequent successful achievement surgical repair or heart transplant; c) there is a temporal trend of increased 1-year survival, although in-hospital mortality rates are still higher than 50%.
Despite all current advancements in AMI treatment, mortality rates of postinfarction VSR are still extremely high, at around 60% in our series and in other registries.2 Our data are similar to previously published data, with a prevalence of VSR of less than 1% of all AMI.3 We observed no temporal changes in patient characteristics, except for younger age in the last 5-year period. We have no clear explanation for the increase in the number of patients in the second half and decrease in the age of presentation of the patients in this cohort. It might be related to less thorough investigation with bedside echocardiogram during the first years of this retrospective analysis or missing diagnosis of this AMI-related mechanical complication in our database, which was composed of prospectively collected data from several hospitals. This finding is intriguing and could be related to the implementation of highly active primary PCI networks in some of the included centers, beginning in 2013 to 2014 (table 1 the supplementary data) with an increase in the total number of ST-segment elevation myocardial infarction patients treated in larger institutions. However, we believe this aspect should be explored in a larger, preferably nationwide study.
Analysis of clinical aspects of the ischemic event showed that apical location of VSR was more frequent, as previously described, with a similar distribution between right and left coronary artery infarctions. The delay in the diagnosis is also important, this issue being a reiterated and classic problem for mechanical complications of AMI. Like previous literature, our study demonstrates that post-AMI VSR is more frequent in patients who delay receiving medical attention during symptom onset, and in a substantial number of patients, the VSR occurs once the patients are in the hospital.
We believe that despite initial symptoms at the time of diagnosis, rapid decline in the hemodynamic status of these patients should be anticipated and elicit a rapid response based on application of mechanical circulatory support therapies. VA-ECMO support, with or without intra-aortic balloon pump, is the preferred option in most centers.17,18 Stabilization with VA-ECMO could potentially favor best overall conditions toward a timely definitive surgical repair. In our study, the increased trend to VA-ECMO support was associated with a slightly better survival in the univariate analysis.
Better repair techniques of the septal defect are still needed. Percutaneous closure has been described with different devices, but the high residual or recurrence of the left-to-right shunting are both associated with very poor overall results, as presented in our study.19–23 Surgical repair is usually the preferred option but is limited by the need for large patches to overcome the fragility of VSR borders, leading to small and/or restrictive left ventricular cavities and refractory cardiogenic shock in some cases. Given the overall poor results of percutaneous and surgical options, it is noteworthy that heart transplant was used as a first-line strategy in some of our centers with good results (survival in 5 out of 6 patients). Heart transplant is not available in all included centers but transferring patients to specific advanced heart failure units is relatively common and feasible within the Spanish health system, even for sick patients such as those that are considered possible candidates. Despite the well-known limitations of the shortage of donors, patient suitability and logistic complexity, heart transplant may be the best option in specific subgroups, such as those with severe and irreversible right/left ventricular failure or extensive defects.
Another important question is the timing between the use of VA-ECMO and definitive surgical repair. From one perspective, allowing time for definitive scarring of VSR borders could theoretically facilitate surgical repair sutures.14 However, prolonged support is associated with more vascular and bleeding complications.17,18,24 Some studies observed that the real benefit of a delayed surgical repair appears 7 days after diagnosis.25 Ariza-Solé et al.14 suggest that surgery should be delayed until the recovery of multiorgan failure, 3 to 4 days after placement of ECMO, and the device should be withdrawn 2 to 3 days after surgical repair when the patient is completely stabilized. Malhotra et al.26 have proposed a prognostic scoring system to establish the optimal timing of surgical repair. In the absence of randomized clinical trials, large observational data such as the present study suggest a benefit of an VA-ECMO-based strategy as a bridge to final reparative surgery or heart transplant. We observed a nonsignificant trend in delayed repair in patients with VA-ECMO, increasing the mean time to repair from 2 to 5 days. In patients treated with VA-ECMO, the optimal timing to surgery appears from the fourth day, with an in-hospital mortality of 36%. This information is congruent with the above-mentioned previous studies.14
One noteworthy finding is that we identified 3 patients in which the cardiac catheterization showed nonobstructive coronary arteries. MINOCA had been previously identified as an etiology of VSR in several clinical cases.27–30 The appearance of this complication in MINOCA seems to be exceedingly rare, and its pathophysiologic features are poorly understood.
This study has several limitations. The observational and retrospective character of our registry, which is supported with historical data from the collaborating centers, is a potential source of selection bias and is the main limitation. The original study design does not allow strong causal associations to be established. This design can favor the loss of data in patients with VSR and early cardiogenic shock, who died in the first few hours of receiving medical attention and could possibly have been excluded from the databases of individual units. In the case of the time to PCI, we do not have information about the exact time until the procedure in hours, which could better explain the incidence of VSR related to a delay in treatment. We did not use an external quality assurance resource for the analysis of these data. However, all selected centers have a prospectively collected database, which should have helped minimize loss of relevant information. The incidence of VSR is an estimated value taken from reports of voluntary participating centers, and therefore should be interpreted with caution. Finally, the contribution of only large centers in this database could limit extrapolation of the prevalence or clinical manifestations of VSR to other settings, although we believe it had a limited impact in the analysis of our primary endpoint and the present data should be viewed in the context of similar tertiary centers.
CONCLUSIONSPost-AMI VSR still carries high in-hospital and 1-year mortality. There is a temporal trend toward a decrease in the mortality of post-AMI VSR that appears to be multifactorial. Management differences among 2 time periods included an increase in use of VA-ECMO support and in heart transplant as a possible alternative in anecdotal cases. The contribution of VA-ECMO to overall survival should be addressed in appropriate clinical trials.
FUNDINGThe authors received no financial support for the research, authorship, and/or publication of this article.
AUTHORS’ CONTRIBUTIONSM. Sanmartín Fernández developed the original project and the hypothesis along with the design and objectives and revised the final manuscript. J.D. Sánchez Vega, G.L. Alonso Salinas, J.M. Viéitez Flórez and J.L. Zamorano Gómez contributed to the analyses of the data and writing of the manuscript. The rest of the authors, including those already listed, contributed to the recruitment process. All authors critically revised the article for important intellectual content and have contributed to the work and agreed on the final version.
CONFLICTS OF INTERESTThe authors declare no competing interests.
AcknowledgmentsCollaboration in data collection: Nagore Horrillo Alonso and Jorge Díaz Calvo from Hospital Universitario de Cruces (Baracaldo), Sandra Rosillo from Hospital Universitario la Paz, IDIPAZ, Madrid.
- –
VSR following a myocardial infarction is a rare but severe mechanical complication with a high mortality rate.
- –
Urgent surgery is the treatment of choice but carries a high mortality rate and appropriate timing is challenging.
- –
Some patients are managed with mechanical circulatory support as bridge therapy to definitive repair. The specific contribution of these aggressive support therapies to survival is not clearly understood.
- –
This study is a large and contemporary registry of VSR that updates the information on important clinical features and management strategies.
- –
We observed an increase in survival among patients with postinfarction VSR in the last decade.
- –
VA-ECMO is being increasingly used in the management of postinfarction VSR.
- –
There is an urgent need to evaluate the role of mechanical support strategies, such as VA-ECMO, as a bridge to definitive repair or heart transplant.
The authors would like to thank Sara Rosenstone Calvo, M.D. for the help provided in editing this manuscript.
Juan Diego Sánchez Vega, Marcelo Sanmartín Fernández, Gonzalo Luis Alonso Salinas, José María Viéitez Flórez, José Luis Zamorano Gómez, Albert Ariza-Solé, Esteban López de Sá, Ricardo Sanz Ruiz, Virginia Burgos Palacios, Sergio Raposeiras-Roubín, Susana Gómez Varela, Juan Sanchis, Lorenzo Silva Melchor, Xurxo Martínez-Seara, Nagore Horrillo Alonso, Jorge Díaz Calvo, Lorena Malagón López, María Luisa Blasco, Andrea Izquierdo Marquisa, Sandra Rosillo.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.07.010