The DECADE cooperation is a pooled analysis of individual patient data from drug-eluting stent (DES) trials with a 10-year follow-up. This analysis reports the risk of definite stent thrombosis (ST) through to 10 years after percutaneous coronary intervention (PCI) in patients treated with early- and new-generation DES.
MethodsIndividual patient data from 5 DES trials with a 10-year follow-up were pooled. The primary endpoint was definite ST up to 10 years after PCI. Patients were divided into 2 groups as per the generation of DES implanted (early and new DES). Individual participant data were analyzed using a 1-stage approach.
ResultsWe included 9700 patients, 6866 in the new DES group and 2834 in the early DES group. Through to 10 years, definite ST occurred in 69 of 6866 patients treated with new DES and in 91 of 2834 patients treated with early DES (1.0% vs 3.5%, adjusted hazard ratio, 0.32; 95%CI, 0.23-0.45). The rate of definite ST was lower in the new DES group than in the early DES group from 1 to 5 years (rate ratio, 0.14; 95%CI, 0.08-0.26) and from 5 to 10 years (rate ratio, 0.23; 95%CI, 0.08-0.61) after PCI.
ConclusionsThe incidence of definite ST through to 10 years after PCI with new-generation DES was 1%. New-generation DES are associated with a lower 10-year incidence of definite ST than early-generation DES, particularly beyond 1 year after PCI.
Keywords
Stent thrombosis (ST) after percutaneous coronary intervention (PCI) is associated with significant morbidity and mortality.1,2 Drug-eluting stents (DES) have superseded bare metal stents (BMS) in clinical practice3–5 and current guidelines recommend the use of DES in preference to BMS irrespective of clinical or anatomical considerations.6 However, early-generation DES (early DES) were associated with an increased risk of very late ST (VLST) compared with BMS, defined as ST> 1 year after PCI.7,8 The increased risk of VLST appears to have been attenuated with new-generation DES (new DES), although the clinical sequelae of ST in the new DES era remain significant.8–11 Very very late ST (VVLST), defined as ST occurring> 5 years after PCI, has also been reported,12 although the risk of VVLST after PCI with early and new DES has not yet been fully defined. Most studies lack sufficient statistical power to assess this endpoint and few studies have follow-up to 10 years after PCI.
We performed the current analysis to define the temporal patterns of ST in patients treated with new and early DES in randomized trials with a 10-year follow-up after PCI.
METHODSThe DECADE cooperationThe Adverse Events and Coronary Artery Disease Progression (DECADE) cooperation is a pooled analysis of individual patient data from DES trials with 10-year follow-up.
We searched for randomized trials investigating 10-year clinical outcomes after DES implantation, across relevant electronic scientific databases. The final search was performed in October 2020. We identified 6 trials with full-length manuscripts.13–18 No further citations were identified after inspection of the reference lists from these eligible studies. The principal investigators of the studies were contacted to provide the individual data of participants assigned to DES implantation. One randomized trial was excluded, as the principal investigator did not agree to share patient-level data.14 Data from the remaining studies were transferred without patient identifiers and combined in a single pooled database. The included trials were ISAR TEST 4,19 ISAR TEST 5,20 SORT OUT III,21 SIRTAX22 and EXAMINATION.23 The final dataset was checked for completeness and consistency and compared with the results from prior publications. Principal investigators were directly contacted if there were inconsistencies with the original publications or requirements for additional data. Divergences were resolved by consensus. Data were analyzed according to the intention-to-treat principle.
Description of DECADE trialsThe ISAR TEST 4 trial randomized 2603 patients to 3 DES treatment arms: new-generation, biodegradable-polymer sirolimus-eluting stents (SES) (n=1299), new-generation, permanent-polymer everolimus-eluting stents (EES) (n=652) and early-generation, permanent-polymer SES (n=652). The ISAR TEST 5 trial randomized 3002 patients to either new-generation, polymer-free sirolimus/probucol-eluting stents (n=2002) or new-generation, permanent-polymer zotarolimus-eluting stents (n=1000). The SIRTAX trial randomized 1012 patients to early-generation, permanent-polymer SES (n=503) or paclitaxel-eluting stents (n=509). The SORT OUT III trial randomized 2332 patients to new-generation, permanent-polymer zotarolimus-eluting stents (n=1162) or early-generation, permanent-polymer SES (n=1170). The EXAMINATION trial randomized 1498 patients to receive either a new-generation, permanent-polymer EES (n=751) or BMS (n=747). Ten-year results of the 5 individual trials included in the DECADE cooperation have already been published.13,15–18 The characteristics of the included trials are summarized in table 1. The key inclusion and exclusion criteria, primary endpoints and definitions of ST for the trials in the DECADE cooperation are summarized in . Each study included in the present analysis was approved by the institutional review board or ethics committee at each participating center, and all patients signed informed, written consent before receiving the assigned treatment.
Characteristics of the trials included in the DECADE cooperation
| Trial name(enrollment period) | DES type; brand name(manufacturer) | Patients/treatment arm | Patients withACS at admission | Patients withdiabetes mellitus | DAPT regimenaccording totrial protocol | Patients with complete 10-year follow-up |
|---|---|---|---|---|---|---|
| SIRTAX(2003-2004) | Early-generation, permanent polymer SES; Cypher Select/Cypher Select Plus (Cordis, Johnson & Johnson, United States) | 503 | 520/1012(51.4) | 201/1012(19.9) | Aspirin 100mg once daily indefinitely; clopidogrel 75mg once daily for 12 mo | 895/1012(88.4) |
| Early-generation, PES; Taxus (Boston Scientific Corp Natick, United States) | 509 | |||||
| ISAR TEST 4(2007-2008) | New-generation, biodegradable-polymer SES | 1299 | 1060/2603(40.7) | 753/2603(28.9) | Aspirin 100mg once daily indefinitely; clopidogrel 150mg for the first 3 d (or until discharge), clopidogrel 75mg once daily for ≥6 mo | 2153/2603(82.7) |
| Early-generation, permanent polymer SES; Cypher (Cordis, Johnson & Johnson, United States) | 652 | |||||
| New-generation, permanent-polymer EES; Xience V (Abbott Vascular, United States) | 652 | |||||
| SORT OUT III(2006-2007) | New-generation, permanent-polymer ZES; Endeavor (Medtronic Cardiovascular, United States) | 1162 | 1052/2332(45.1) | 337/2332(14.5) | Aspirin 75mg once daily indefinitely; clopidogrel 75mg once daily for 12 mo | 2312/2332(99.1) |
| Early-generation, permanent polymer SES; Cypher Select/Cypher Select Plus(Cordis, Johnson & Johnson, United States) | 1170 | |||||
| ISAR TEST 5(2008-2009) | New-generation, polymer-free sirolimus- and probucol-eluting stent; ISAR VIVO (Translumina Therapeutics, Germany) and Coroflex ISAR (B. Braun Melsungen, Germany) | 2002 | 1232/3002(41.0) | 870/3002(29.0) | Aspirin 100mg once daily indefinitely; clopidogrel 150mg for the first 3 d (or until discharge), clopidogrel 75mg once daily for ≥6 mo | 2553/3002(85.0) |
| New-generation, permanent-polymer ZES; Resolute (Medtronic Cardiovascular, United States) | 1000 | |||||
| EXAMINATION(2008-2010) | New-generation, permanent-polymer EES; Xience V (Abbott Vascular, United States) | 751 | 751/751(100) | 137/751(18.2) | Aspirin 100mg once daily indefinitely; clopidogrel 75mg once daily for 12 mon | 710/751(94.5) |
ACS, acute coronary syndrome; DAPT, dual antiplatelet therapy; DES, drug-eluting stents; EES, everolimus-eluting stents; PES, paclitaxel-eluting stents; SES, sirolimus-eluting stents; ZES, zotarolimus-eluting stent.
The data are presented as absolute numbers or No. (%).
The primary endpoint of the current analysis was definite ST through to 10 years after PCI. Information regarding probable ST was not included in all trials and so we were unable to include this as an endpoint. For the analysis of this endpoint, patients were divided into 2 groups as per the generation of DES implanted, namely early DES and new DES. Stents included in the early DES group were permanent-polymer SES and paclitaxel-eluting stents. Stents included in the new DES group were permanent-polymer zotarolimus-eluting stents, biodegradable-polymer SES, permanent-polymer EES and polymer-free sirolimus/probucol-eluting stents. Data from the group of patients randomized to BMS in the EXAMINATION trial were excluded from this analysis, as they were not considered relevant to the study research question, which was focused on ST after DES implantation. In the SORT OUT III trial, ST data were available up to 5 years of follow-up. All endpoints were evaluated according to the definitions in the original trial protocols (). ST was defined according to the Academic Research Consortium criteria in 4 of 5 trials included in the DECADE cooperation (ISAR TEST 4, ISAR TEST 5, SORT OUT III and EXAMINATION19–21,23). In the SIRTAX trial, definite ST was adjudicated in cases of acute coronary syndrome with angiographic documentation of either occlusion of the target lesion or thrombus within the previously stented segment.22 The definitions of ST used in the trials are summarized in . We also provided data regarding mortality and myocardial infarction in patients treated with new- and early-generation DES, in order to provide more context to the definite ST results.
Statistical analysisIndividual participant data were analyzed using a 1-stage approach. Continuous data are presented as means±standard deviation or medians and interquartile ranges. Categorical data are presented as counts and proportions. Data distribution was tested for normality by using the Kolmogorov-Smirnov test for goodness-of-fit. Differences between groups were checked for significance using an analysis of variance test (ANOVA) for continuous data. Depending on the data distribution, the chi-squared test or Fisher exact test was used to check for differences between categorical variables. Here, a 2-tailed P value of <.05 was taken to confer statistical significance. Survival was analyzed by the Kaplan-Meier method to estimate the time to first event for the endpoint of interest and differences between the 2 groups were tested with the log-rank test. Hazard ratios (HR) and 95% confidence intervals (95%CI) were calculated using a Cox proportional hazards model after checking for fulfilment of the proportional hazards assumption as per the method of Grambsch and Therneau.24 The analysis of ST accounted for the competing risk of death using the cuminc function in the cmprsk package in R. Adjusted hazard ratios (HRadjusted) with pertinent 95%CI were reported. These were derived from a conventional multivariable analysis with adjustment for the following variables: age, sex, diabetes mellitus, hypertension, smoking, hypercholesterolemia, history of myocardial infarction, acute coronary syndrome, and vessel treated. We also performed a multivariate sensitivity analysis, which accounted for several angiographic and procedural variables in addition to patient characteristics with <5% missing values in the pooled dataset, including age, sex, diabetes, hypertension, smoking status, hypercholesterolaemia, previous myocardial infarction, acute coronary syndrome presentation, treated vessel, lesion complexity, balloon diameter and total stented length.
We performed a landmark analysis for ST for the following time periods: 0 to 30 days (accounting for acute and subacute ST), 30 days to 1 year (accounting for late ST), 1 to 5 years (accounting for VLST) and 5 to 10 years (accounting for VVLST). ST event rates were also calculated for these time periods, compared using the exact 2-sided Poisson test and expressed as a rate ratio (RR) and 95%CI. For ST from 0 to 30 days after PCI, rates of ST were expressed as the number of events per 1000 patient days of follow-up. From 30 days to 1 year, 1 to 5 years and 5 to 10 years after PCI, the ST rate was expressed as the number of events per 1000 patient years of follow-up. The statistical analysis was performed using the R 3.6.0 Statistical Package (The R Foundation for Statistical Computing, Austria).
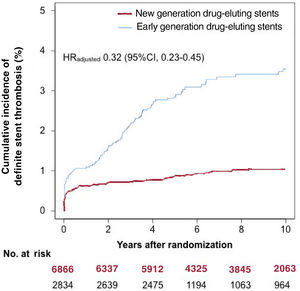
RESULTSPooling of the individual trial populations led to the inclusion of 9700 patients in this analysis. Of these patients, 6866 were treated with new DES and 2834 were treated with early DES. After PCI, all patients were prescribed a dual antiplatelet therapy regimen consisting of aspirin ≥ 75mg daily indefinitely and clopidogrel 75mg daily for a minimum of 6 months and up to 12 months after PCI. Details of the antiplatelet regimens used in each trial are provided in table 1. The median follow-up [25th, 75th percentiles] among survivors was 10.0 [9.9, 10.9] years. Only 698 of the 9700 patients (7.2%) had a follow-up shorter than 9 years.
Baseline characteristicsTable 2 displays the baseline characteristics for the cohort as a whole and as per the stent generation implanted. Patients in the new DES group were older and were more frequently diabetic and hypertensive. Current smokers at the time of PCI were more frequent in the early DES group. In over half of patients in both the early DES and new DES groups, the indication for PCI was stable angina. Table 3 displays the baseline angiographic and procedural characteristics both for the cohort as whole and as per the stent generation implanted. There was a higher proportion of lesions with complex morphology and treated bifurcation lesions in the new DES group. In addition, the new DES group had a longer total stented length and a higher total number of implanted stents than the early DES group.
Baseline characteristics as per drug-eluting stent generation*
| Characteristic | All DES(n=9700) | New DES(n=6866) | Early DES(n=2834) | P |
|---|---|---|---|---|
| Trials | ||||
| EXAMINATION | 751 (7.7) | 751 (10.9) | 0 (0) | |
| ISAR TEST 4 | 2603 (26.8) | 1951 (28.4) | 652 (23.0) | |
| ISAR TEST 5 | 3002 (30.9) | 3002 (43.7) | 0 (0) | |
| SIRTAX | 1012 (10.4) | 0 (0) | 1012 (35.7) | |
| SORT OUT III | 2332 (24.0) | 1162 (16.9) | 1170 (41.3) | |
| Age, y | 65.5±11.3 | 66.1±11.3 | 64.1±11.1 | <.001 |
| Female | 2296 (23.7) | 1600 (23.3) | 696 (24.6) | .195 |
| Diabetes mellitus | 2298/9699 (23.7) | 1736/6865 (25.3) | 562 (19.8) | <.001 |
| Insulin-dependent | 638 (6.6) | 536 (7.8) | 102 (3.6) | <.001 |
| Hypertension | 5923/9609 (61.6) | 4293/6824 (62.9) | 1630/2785 (58.5) | <.001 |
| Current smoker | 2365/9527 (24.8) | 1545/6783 (22.8) | 820/2744 (29.9) | <.001 |
| Hypercholesterolemia | 6110/9613 (63.6) | 4333/6827 (63.5) | 1777/2786 (63.8) | .789 |
| Body mass index, kg/m2 | 27.4±4.4 | 27.5±4.5 | 27.3±4.3 | .036 |
| Prior myocardial infarction | 2547/9598 (26.5) | 1766/6816 (25.9) | 781/2789 (28.1) | .031 |
| Number of diseased coronary vessels | <.001 | |||
| One vessel | 2258/7368 (30.6) | 1327 (23.3) | 931/1664 (55.9) | |
| Two vessels | 1798/7368 (24.4) | 1463 (25.6) | 335/1664 (20.1) | |
| Three vessels | 3297/7368 (44.7) | 2914 (51.1) | 383/1664 (23.0) | |
| Number of lesions | 1.4±0.6 | 1.4±0.6 | 1.4±0.6 | .742 |
| Clinical presentation | .001 | |||
| Acute coronary syndrome | 4557 (47.0) | 3299 (48.0) | 1258 (44.4) | |
| Stable angina | 5143 (53.6) | 3567 (52.0) | 1576 (55.6) | |
| Ejection fraction, % | 53.3±11.7 | 52.6±11.6 | 55.6±12.0 | <.001 |
DES, drug-eluting stents.
Data are expressed as No. (%) or mean±standard deviation.
Angiographic and procedural characteristics as per drug-eluting stent generation
| All DES | New DES | Early DES | P | |
|---|---|---|---|---|
| Lesions | (n=13 180) | (n=9320) | (n=3860) | |
| Target vessel | <.001 | |||
| Left main coronary artery | 80 (0.6) | 29 (0.3) | 51 (1.3) | |
| Left anterior descending coronary artery | 5791 (43.9) | 4118 (44.2) | 1673 (43.3) | |
| Left circumflex coronary artery | 3234 (24.5) | 2283 (24.5) | 951 (24.6) | |
| Right coronary artery | 4023 (30.5) | 2873 (30.8) | 1150 (29.8) | |
| Bypass graft | 48 (0.4) | 14 (0.2) | 34 (0.9) | |
| Bifurcation involved and treated | 2145/9172 (23.4) | 1831/6924 (26.4) | 314/2248 (14.0) | <.001 |
| Complex lesion (type B2/C) | 7804/12 343 (63.2) | 5882/8520 (69.0) | 1922/3823 (50.3) | <.001 |
| Preprocedural reference vessel diameter, mm | 2.8 (2.44-3.1) | 2.8 (2.4-3.1) | 2.8 (2.5-3.1) | .066 |
| Preprocedural minimal lumen diameter, mm | 0.9 (0.6-1.2) | 0.9 (0.6-1.2) | 0.7 (0.3-1.0) | <.001 |
| Balloon diameter, mm | 3.0 (2.8-3.5) | 3.1 (2.7-3.5) | 3.0 (2.8-3.5) | <.001 |
| Maximal balloon pressure, atm | 16.0 (12.2-18.0) | 16.0 (13.0-18.0) | 14.0 (12.0-16.3) | <.001 |
| Total stented length, mm | 18.0 (16.0-28.0) | 23.0 (18.0-30.0) | 18.0 (13.0-23.0) | <.001 |
| Number of stents | 1.0 (1.0-2.0) | 2.0 (1.0-2.0) | 1.0 (1.0-1.0) | <.001 |
| Postprocedural minimal lumen diameter, mm | 2.6 (2.2-2.9) | 2.6 (2.2-2.9) | 2.6 (2.3-2.9) | .414 |
| Postprocedural diameter stenosis, % | 10.7 (7.3-14.9) | 11.0 (7.6-15.1) | 9.5 (6.0-13.5) | <.001 |
DES, drug-eluting stent.
Data are expressed as median [interquartile range] or No. (%).
* Completeness of continuous data: preprocedural reference vessel and minimal lumen diameter were not available for 4103 lesions (1692 in the early DES group and 2411 in the new DES group); balloon diameter was not available for 159 lesions (63 in the early DES group and 96 in the new DES group); maximal balloon pressure was not available for 4526 lesions (2104 in the early DES group and 2422 in the new DES group); total stented length was not available for 67 lesions (21 in the early DES group and 46 in the new DES group); number of stents was not available for 417 lesions (216 in the early DES group and 201 in the new DES group); postprocedural minimal lumen diameter and diameter stenosis was not available for 4865 lesions (2447 in the early DES group and 2418 in the new DES group). The remaining data are complete.
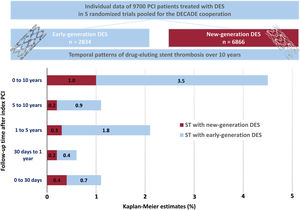
Definite ST occurred in 160 of 9700 patients (1.6%) through to 10-year follow-up. The incidence of definite ST through to 10 years as per each individual stent type is shown in . In the new DES group, definite ST occurred in 69 of 6866 patients (1.0%) through to 10-year follow-up. In the early DES group, definite ST occurred in 91 of 2834 patients (3.5%) through to 10-year follow-up. The cumulative incidence of definite ST through to 10 years was lower in patients treated with new DES than in those treated with early DES in both the unadjusted (HR, 0.30; 95%CI, 0.22-0.41) and adjusted (HRadjusted, 0.32; 95%CI, 0.23-0.45) analyses. These data are presented in figure 1 and table 4.
Ten-year cumulative incidence of definite stent thrombosis as per drug-eluting stent generation. The hazard ratio reported here was derived from a conventional multivariable analysis with adjustment for the following variables: age, sex, diabetes mellitus, hypertension, smoking, hypercholesterolemia, history of myocardial infarction, acute coronary syndrome, and vessel treated. 95%IC, 95% confidence interval; HR, hazard ratio.
Definite stent thrombosis through to 10 years as per drug-eluting stent generation
| Definite stent thrombosis | NewDES(n=6866) | EarlyDES(n=2834) | Unadjustedhazard ratio[95%CI] | Adjustedhazard ratio[95%CI] |
|---|---|---|---|---|
| 0 to 10 y | 69 (1.0) | 91 (3.5) | 0.30 [0.22-0.41] | 0.32 [0.23-0.45] |
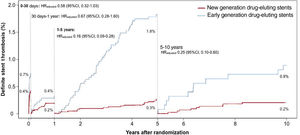
| 0 to 30 d | 29 (0.4) | 21 (0.7) | 0.57 [0.32-0.99] | 0.58 [0.32-1.03] |
| 30 d to 1 y | 14 (0.2) | 10 (0.4) | 0.58 [0.26-1.30] | 0.67 [0.28-1.60] |
| 1 to 5 y | 17 (0.3) | 49 (1.8) | 0.14 [0.08-0.25] | 0.16 [0.09-0.28] |
| 5 to 10 y | 9 (0.2) | 11 (0.9) | 0.23 [0.10-0.56] | 0.25 [0.10-0.60] |
95%CI, 95% confidence interval; DES, drug-eluting stent.
Data are shown as number of events with Kaplan-Meier estimates (%) for primary endpoint (definite stent thrombosis) after accounting for competing risk for death. The adjusted hazard ratio reported here was derived from a conventional multivariable analysis with adjustment for the following variables: age, sex, diabetes mellitus, hypertension, smoking, hypercholesterolemia, history of myocardial infarction, acute coronary syndrome, and vessel treated.
Figure 2 and table 4 demonstrate the incidence of definite ST within 4 time-periods: 0 to 30 days, 30 days to 1 year, 1 to 5 years, and 5 to 10 years. Compared with the early DES group, the incidence of definite ST was lower in the new DES group from 1 to 5 years (0.3% vs 1.8%, HRadjusted, 0.16; 95%CI, 0.09-0.28) and from 5 to 10 years (0.2% vs 0.9%, HRadjusted, 0.25; 0.10-0.60) after PCI.
Definite stent thrombosis: event rate analysisFrom 0 days to 30 days after PCI, the definite ST rate was 0.14 (95%CI, 0.10-0.20)/1000 patient days in the new DES group and 0.25 (95%CI, 0.15-0.38)/1000 patient days in the early DES group (RR, 0.57; 95%CI, 0.31-1.05). From 30 days to 1 year after PCI, the definite ST rate was 2.30 (95%CI, 1.26-3.85)/1000 patient years in the new DES group and 3.97 (95%CI, 1.90-7.29)/1000 patient years in the early DES group (RR, 0.58; 95%CI, 0.24-1.46). From 1 to 5 years, the definite ST rate was 0.69 (95%CI, 0.40-1.11)/1000 patient years in the new DES group and 4.80 (95%CI, 3.55-6.34)/1000 patient years in the early DES group (RR, 0.14; 95%CI, 0.08-0.26). From 5 to 10 years, the definite ST rate was 0.46 (95%CI, 0.21-0.87)/1000 patient years in the new DES group and 1.99 (95%CI, 0.99-3.57)/1000 patient years in the early DES group (RR, 0.23; 95%CI, 0.08-0.61). These data are presented in table 5.
Definite stent thrombosis rate as per drug-eluting stent generation and time after PCI
| Time period(unit of rate) | Definite stent thrombosis event rate (95%CI) | Rate ratio(95%CI) | |
|---|---|---|---|
| New DES(n=6866) | Early DES(n=2834) | ||
| 0 to 30 d(per 1000 patient d) | 0.14 (0.10-0.20) | 0.25 (0.15-0.38) | 0.57 (0.31-1.05) |
| 30 d to 1 y(per 1000 patient y) | 2.30 (1.26-3.85) | 3.97 (1.90-7.29) | 0.58 (0.24-1.46) |
| 1 to 5 y(per 1000 patient y) | 0.69 (0.40-1.11) | 4.80 (3.55-6.34) | 0.14 (0.08-0.26) |
| 5 to 10 y(per 1000 patient y) | 0.46 (0.21-0.87) | 1.99 (0.99-3.57) | 0.23 (0.08-0.61) |
95%CI, 95% confidence interval; DES, drug-eluting stent; PCI, percutaneous coronary intervention.
In the multivariate sensitivity analysis (see Methods for details), new DES were also associated with a reduced risk of definite ST than early DES at 10 years (HR, 0.27; 95%CI, 0.19-0.39). The reduced risk of definite ST with new DES was confirmed in this model from 0 to 1 years (HR, 0.57; 95%CI, 0.34-0.94) and from 1 to 10 years (HR, 0.11; 95%CI, 0.06-0.21).
Additional endpoints: mortality and myocardial infarctionDuring follow-up, death occurred in 2004 of 6866 patients (30.4%) in the new DES group and in 765 of 2834 patients (28.3%) in the early DES group (HRadjusted, 0.97; 95%CI, 0.89-1.06). Myocardial infarction occurred in 461 of 6866 patients (6.9%) in the new DES group and in 291 of 2834 patients (10.7%) in the early DES group (HRadjusted, 0.66; 95%CI, 0.57-0.77). At 10 years, 31 of 74 patients who had experienced a definite ST event at 1 year died compared with 2738 of 9626 patients who did not experience definite ST at 1 year (41.9% vs 28.4%, P=.01).
DISCUSSIONThe main findings of this study are as follows (figure 3): a) the cumulative incidence of definite ST through to 10 years after PCI with new DES was 1%; b) new DES are associated with a lower 10-year cumulative incidence of definite ST than early DES; c) new DES are particularly associated with a reduction in the rate of VLST and VVLST compared with early DES.
In this analysis, new DES were associated with a lower 10-year incidence of ST than early DES. However, it is important to consider that across the trials included in the DECADE cooperation, participants were not always randomized to either early or new DES. As such, there were some differences in baseline and procedural characteristics between the 2 groups. Patients treated with new DES were more frequently diabetic, with a higher proportion of treated bifurcation lesions, more complex lesion morphology and a longer stented length than patients treated with early DES. All these factors have been associated with an increased risk of ST.25–28 Despite this, the new DES group in our study was associated with a lower incidence of definite ST through to 10 years on both unadjusted and adjusted analyses. This was particularly apparent during the period from 1 to 5 years after PCI. Concerns regarding VLST had tempered initial enthusiasm for early DES.29 The findings of the current analysis are reassuring and suggest that iterative improvements in DES technology have mitigated this risk.
Several factors may have contributed to the observed superiority of new DES over early DES in this analysis. These include improvements in stent design, reductions in stent strut thickness, and novel polymer technologies. The type, quantity and release kinetics of the eluted antiproliferative drugs may also have played an important role. While it may be tempting to view the lower incidence of ST associated with new DES compared with early DES in our analysis as a class effect, it is important to remember that there were also differences between the stent technologies included within both the early and new DES groups.
In recent years, cases of VVLST have been reported in the scientific literature, defined as ST occurring> 5 years after stent implantation.12 Reassuringly, the current analysis suggests that the risk of VVLST is lower in new DES than in early DES.
There are no previous publications comparing the incidence of ST through to 10 years after PCI with early DES and new DES. However, there are some previous observational analyses with shorter durations of follow-up. Our group reported that compared with BMS, early DES were associated with an increased risk of ST from 1 year up to 3 years after PCI, while BMS and new DES were associated with a similar risk of ST.8 A meta-analysis of trials with follow-up periods ranging from 1 to 5 years after PCI suggested that EES may reduce the risk of ST compared with early DES.30 Similarly, network meta-analyses and a randomized head-to-head comparison have reported that EES significantly reduced ST at 1 year compared with paclitaxel-eluting stents.31–33 The 10-year duration of follow-up in our analysis may have allowed time for important differences to emerge between the new and early DES groups.
The new DES cohort in this analysis was a heterogeneous group and included biodegradable-polymer, permanent-polymer and polymer-free DES platforms. Previous meta-analyses have suggested that the frequency of VLST is comparable between new-generation, permanent-polymer DES and biodegradable-polymer DES, as well as between new-generation, permanent-polymer DES and polymer-free DES.34,35 It has been suggested that new DES with ultrathin stent backbones may further reduce the risk of ST compared with new DES with thicker backbones, although the current evidence in this regard is limited.36,37
In patients treated with new DES in the current analysis, the highest incidence of definite ST with new DES occurred within the first 30 days after PCI. The rate of definite ST was markedly lower with new DES than with early DES from 1 to 5 years and from 5 to 10 years after PCI, suggesting that the risk of both VLST and VVLST has been reduced with improvements in DES technology. These data may have relevance for proposed pharmaco-therapeutic strategies after new DES implantation, including prolonged antiplatelet therapy regimens, as well as for future DES trial design. In the first case, the potential benefits of prolonged antiplatelet regimens may not be as apparent for patients treated with new DES, which lends support to the concept of de-escalation.38 In the second case, given that the long-term incidence of definite ST after PCI with new DES was 1% through to 10-year follow-up, a large number of patients would be required for a future study to have adequate statistical power to demonstrate a meaningful reduction in definite ST compared with this standard. From a pragmatic perspective, this suggests that it will be challenging for future studies to demonstrate the superiority of newer stent technologies with respect to this endpoint.
LimitationsThis is a post hoc analysis of individual patient data from 5 RCTs. As such, it has the usual limitations associated with post hoc analyses and should be regarded as hypothesis generating. For the purposes of this analysis, stents implanted were categorized as early and new DES. However, there are differences between the stent platforms within these 2 categories and therefore this dichotomization could be considered overly reductionist or arbitrary. Of interest, the incidence of definite ST between the individual stent types grouped in the new and early DES cohorts appeared to support this dichotomization. Nevertheless, the present analysis is largely underpowered to assess differences in terms of ST within the new and early DES groups. In addition, patients were not randomized in all the included studies to treatment with either early or new DES and there were some differences in baseline and procedural features between the 2 groups. In light of these arguments, although risk estimates for the primary endpoint went in the same direction on both unadjusted and adjusted analyses, a potential bias due to residual confounding cannot be definitively ruled-out in this context. The risk of ST is determined not only by stent-related factors but also by other factors, including iteration of implantation techniques, patient selection and periprocedural and long-term antithrombotic regimens over time. All these factors may also have varied between studies and contributed to the observed differences.
Another potential limitation is that we did not have detailed information on long-term antiplatelet therapy or secondary prevention measures for patients enrolled in the original studies. It is also important to consider that the PCI procedures performed in the trials included in the DECADE cooperation may not reflect current practice. While event adjudication processes were similar between all trials included in the DECADE cooperation, events were not adjudicated centrally in this analysis and therefore we cannot exclude heterogeneity with regard to the reporting and adjudication of events between the individual trials.
Another important point is that the patients enrolled in the trials included in the DECADE cooperation represented a selected cohort and therefore may not be fully representative of patients encountered in clinical practice. Given that this analysis focused only on definite ST in a relatively selected cohort of PCI patients, it may have underestimated the true incidence of ST.
CONCLUSIONSThe incidence of definite ST after PCI with new-generation DES was 1% through to 10 years after PCI. New-generation DES were associated with a lower 10-year incidence of definite ST than early-generation DES, particularly beyond 1 year after PCI.
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSJ.J. Coughlan was principally responsible for drafting the manuscript. A. Aytekin assisted with drafting the manuscript. A. Kastrati was primarily responsible for statistical analysis. SC was primarily responsible for conception of the project. M. Maeng, L. Räber, S. Bär, A. Aytekin, L. Okkels Jensen, S. Brugaletta, L. Ortega-Paz, K-L. Laugwitz, M. Madsen, D. Heg, M. Sabaté, S. Kufner, K.K. Warnakula Olesen, and S. Windecker all provided editorial revision to the first draft as well as working on the databases of the original trials. All authors reviewed the submitted version of the manuscript prior to submission.
J. J. Coughlan and M. Maeng contributed equally.
CONFLICTS Of INTERESTThe authors declare the following conflicts of interest: S. Brugaletta is on the advisory board of Boston Scientific and has received lecture fees from Abbott Vascular. S. Kufner Reports lecture fees from AstraZeneca, Bristol Myers Squib, and Translumina. The other authors have no conflicts of interest relevant to this manuscript to report.
- •
New-generation DES have reduced the risk of very late stent thrombosis (ST) compared with early-generation DES. However, there have been no analyses of ST through to 10 years after PCI in a large number of patients.
- •
In our study, new-generation DES were associated with a lower 10-year incidence of definite ST vs early-generation DES, particularly beyond 1 year after PCI.
The authors would like to acknowledge the efforts of the collaborators who were involved in the conduct of the studies that make up the DECADE collaboration. These include: Nonlag Rifatov, RN; Helle Bargsteen, research coordinator; Christa Schönenberger, RN; Josep Gómez-Lara, MD, PhD; Susanne Pinieck, RN; Víctor Jiménez-Díaz, MD, PhD; Laura Morf, PhD.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2022.02.003