A healthy 59-year-old man experienced sudden cardiac death caused by ventricular fibrillation while jogging in the street. Cardiopulmonary resuscitation was initiated with return of spontaneous circulation after 11minutes and 4 defibrillations. He had no cardiovascular risk factors or family history of heart disease. He had been running 5km daily for more than 5 years and had never had any symptoms.
He was intubated and received mechanical ventilation. Initial blood pressure was 154/110mmHg. Pupils were isochoric and reactive. No myoclonus was observed. The first electrocardiogram showed sinus rhythm at 98 beats per minute, narrow QRS and nonspecific conduction disturbances.
A therapeutic hypothermia protocol was started using the CoolGard 3000 device and Icy catheter. At that moment, the lactate level was 0.9 mmol/L. It took 7hours to reach 33°C. Propofol was used at a rate of 0.4-1.2mg/kg per hour to maintain a bispectral index value between 40 and 60.
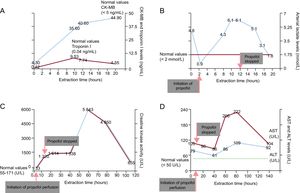
Mean blood pressure was higher than 70mmHg and urine output was about 1.8mL/kg/h. There was a typical rise and fall of plasma creatinine kinase-isoenzyme MB and troponin I (Figure A). A first 24-hour electroencephalogram showed nonspecific diffuse slow activity and response to all stimuli. The first 24-hour serum neuron-specific enolase level was 14.8 ng/mL. At 6hours after propofol infusion, blood analysis revealed a lactate level of 4.3 mmol/L, which peaked at 6.1 mmol/L 4hours later. Due to suspicion of potential propofol toxicity, the drug was discontinued (12hours after initiation of propofol perfusion). Lactate levels dropped rapidly to a normal range 8hours later (Figure B). Creatinine kinase activity increased progressively until reaching a peak of 5843 UI/L 48hours after propofol discontinuation (Figure C). Increased lactate dehydrogenase and transaminase levels were also detected after propofol discontinuation (Figure D).
Changes in the different biochemical parameters of interest and their association with propofol administration. A: CK-MB and troponin I levels. B: arterial lactate levels; C: creatinine kinase activity. D: AST and ALT levels. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK-MB, creatinine kinase-isoenzyme MB.
The patient's clinical course was satisfactory. Coronary angiography was performed, revealing severe 2-vessel disease. A cardiac magnetic resonance scan showed an ejection fraction of 60% with a subendocardial infarct in the territory of the left anterior descending artery. At 3 months, the patient was fully recovered, and gave written consent to this publication.
Increased blood lactate levels may be found in all clinical conditions involving tissular hypoperfusion. The most common type, known as type A lactic acidosis, may be due to conditions that decrease oxygen delivery, usually caused by hypoxemia. Type B lactic acidosis is due to excess demand for oxygen or metabolic problems.
In our patient, systolic heart failure was ruled out due to hemodynamic conditions (mean blood pressure > 70mmHg, urine output > 1.5mL/kg/h spontaneously). Intestinal ischemia could reasonably be ruled out, despite the effects of sedation-analgesia, with a soft abdomen and no fecal incontinence. Sepsis and postcardiac arrest syndrome are scenarios in which increased lactate levels occur. However, this immediate effect on increased lactate rules out these causes. The initially normal creatinine-kinase activity made the findings unlikely due to trauma or compartment syndrome. Renal function was not altered, thereby eliminating acute renal failure as a potential cause. Liver function tests demonstrated mild elevated transaminases with a total bilirubin level and parameters of the blood coagulation system within normal ranges. This pattern does not support hepatocellular failure as a cause of increased lactates in our patient. Finally, generalized convulsions, another cause of raised lactate levels, were not observed.
Propofol-related infusion syndrome (PRIS) is a rare but life-threatening complication in patients receiving propofol. Briefly, the pathophysiological mechanism consists of a failure of adenosine triphosphate production by the inhibitory effect at the mitochondrial electron transport chain and the conversion of free fatty acids to acyl-coA, resulting in an increase in lactic acid and rhabdomyolysis.1 Clinical features consist of severe metabolic acidosis, hyperkalemia, lipidemia, rhabdomyolysis, hepatomegaly, arrhythmias, and cardiac, renal, or circulatory failure. Although PRIS has occurred most frequently in patients receiving high propofol dosages (higher than 4-5mg/kg per hour) and prolonged administration (for more than 48hours), there are reported cases with lower doses or after a shorter duration of sedation.1–4 In our patient, propofol was administered at a low infusion rate (0.4-1.2mg/kg per hour) for less than 12hours. To our knowledge, this is the first case of PRIS described in a patient with hypothermia that appeared in less than 12hours, at very low doses () and without risk factors for developing PRIS, such as steroid or catecholamine use, carbohydrate depletion, young age, or the presence of neurological or neurosurgical diseases.5
The development of an unexplained increase in lactic acid alerted us to a possible adverse effect of propofol. The outcome was good after discontinuation of propofol infusion and the patient made a complete recovery. The strong temporal relationship between propofol administration and the development of the syndrome suggested a potential causal relationship. The therapeutic hypothermia to 33oC used in this patient could be the main factor that boosted the effect of propofol in the development of PRIS. Some studies have shown that propofol clearance is significantly lower during hypothermia compared with normothermia and therefore it can be assumed that, although the propofol infusion rate was clearly below that in previously reported cases, the serum concentration and the effects of propofol were probably higher than expected.
Because propofol is commonly used for sedation in critical care settings in combination with therapeutic hypothermia, it is important to be aware of this possibility and recognize the early signs of PRIS, regardless of the dose and time administered.