Noncompaction cardiomyopathy is characterized by marked ventricular trabeculation. Although its development has been linked to an arrest in normal myocardial compaction during the first trimester of pregnancy,1 it can also be acquired in certain physiological (sport, pregnancy) or pathological (hypertension, anemia) situations or be related to other cardiomyopathies or congenital heart diseases. Diagnosis is based on imaging tests2 and patients can be either asymptomatic or show symptoms of heart failure, arrhythmias, or the effects of thromboembolic phenomena. Factors such as the degree of ventricular dysfunction and dilatation and the presence of late enhancement on cardiac magnetic resonance imaging have been associated with a worse outcome.3 Severe ventricular dysfunction at diagnosis has been described, with partial or complete functional recovery and subsequent deterioration.4
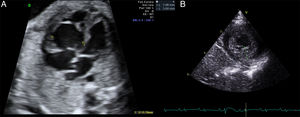
Here, we present the case of a neonate with a prenatal diagnosis of noncompaction cardiomyopathy. The mother had history of fetal death due to hydrops of unknown origin in a previous pregnancy. In this case, at 28 weeks of gestation, a hypertrabeculated right ventricle with severe dysfunction was seen (Figure A), as well as absent antegrade pulmonary flow, severe tricuspid regurgitation, and left ventricular noncompaction with preserved function. Maternal treatment was begun with digoxin until 36 weeks of pregnancy, with echocardiography showing improved right ventricular function.
The patient was born at 39 weeks of pregnancy with good clinical status, and admission echocardiography revealed left ventricular trabeculations (Figure B) with severe left and moderate right ventricular dysfunction (Table). Electrocardiography showed altered repolarization with negative T waves from V3 to V6. Treatment with milrinone was begun and, at 8 days of life, the patient showed slightly improved ventricular function. The newborn was discharged under treatment with angiotensin-converting enzyme inhibitors and beta-blockers.
Echocardiographic Changes Over Time
| Age | LVEDD, z-score | LVEF, % | S’m, cm/s | E/e’ | MR | TAPSE, mm | S’t, cm/s | TR | TR gradient, mmHg |
|---|---|---|---|---|---|---|---|---|---|
| Birth | + 1.8 | 29 | 2 | 23 | Minimal | 6 | 4 | Moderate | 34 |
| High (6 dol) | + 1.8 | 40 | 4 | 16.5 | Mild | 8 | 6 | Mild | 29 |
| Readmission | + 3 | 30 | 4 | 18.8 | Mild | 9 | 7 | Moderate | 40 |
| 48 d | + 3.1 | 27 | 2 | 23.3 | Mild | 12 | 8 | Minimal | 36 |
| 2 mo | + 1.5 | 50 | 3 | 21.8 | Mild | 8 | 17 | Minimal | 35 |
| 3 mo | + 1 | 67 | 4 | 14.6 | Mild | 9 | 11 | Minimal | 27 |
| 9 mo | –0.78 | 66 | 5 | 9 | Minimal | 14 | 9 | Minimal | 25 |
| 2 y | + 1.4 | 64 | 6 | 5.7 | No | 18 | 14 | No |
dol, days of life; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; S’m, mitral S on tissue Doppler; S’t, tricuspid S on tissue Doppler; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
At 34 days of life, the patient had cardiogenic shock and required respiratory and hemodynamic support. Echocardiography showed severe left ventricular dysfunction with retrograde pulmonary hypertension and preserved right ventricular function (Table). Hemodynamic and respiratory support was maintained until the tenth day of admission. The baseline treatment was restarted and digoxin was added. Inclusion of the infant on the heart transplant list was considered but was ruled out due to the patient's clinical and echocardiographic improvement from 2 months of life, with subsequent normalization of ventricular function (Table). After a 2-year follow-up, the patient was asymptomatic and echocardiography showed normalized biventricular systolic and diastolic function. During the clinical course, no arrhythmic or embolic phenomena occurred. Given the functional improvement and subsequent deterioration described in the literature, we decided to continue treatment with angiotensin-converting enzyme inhibitors and beta-blockers.
To determine the cause, the parents and the sister underwent a cardiologic evaluation, which was normal, and all underwent a genetic study. The patient was found to have 2 mutations in the LDB3 gene,5 both described previously as being pathogenic in patients with noncompaction cardiomyopathy. The genetic study of the sister was normal but a mutation was found in both of the patients.
In conclusion, noncompaction cardiomyopathy can be diagnosed during the intrauterine period. It often presents as right ventricular dysfunction during fetal life because this is the dominant ventricle. After birth, with the drop in pulmonary vascular resistance and increase in systemic resistance, there is often improvement in right ventricular function and development of left ventricular dysfunction. Some authors report that the introduction of early medical treatment can improve the outcome and that the response to this treatment might be better than that seen in other cardiomyopathies.6 Accordingly, and given the potential deterioration during the clinical course,4 the therapy was maintained despite the patient's functional recovery. Regarding the family study, a cardiology assessment should be performed in all first-degree relatives. The timing of the genetic study is unclear. In isolated cases of noncompaction cardiomyopathy, genetic study is positive in about 15% to 40% of cases, according to the literature. We believe that genetic study is an important component in the assessment of these patients and that it represents a fundamental tool for obtaining a better understanding of this disease. The identification of new genes involved and the progressive decrease in costs will make it part of standard clinical practice.