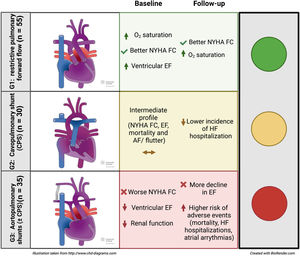
There is scarce information on patients with single ventricle physiology (SVP) and restricted pulmonary flow not undergoing Fontan circulation. This study aimed to compare survival and cardiovascular events in these patients according to the type of palliation.
MethodsSVP patient data were obtained from the databases of the adult congenital heart disease units of 7 centers. Patients completing Fontan circulation or developing Eisenmenger syndrome were excluded. Three groups were created according to the source of pulmonary flow: G1 (restrictive pulmonary forward flow), G2 (cavopulmonary shunt), and G3 (aortopulmonary shunts±cavopulmonary shunt). The primary endpoint was death.
ResultsWe identified 120 patients. Mean age at the first visit was 32.2 years. Mean follow-up was 7.1 years. Fifty-five patients (45.8%) were assigned to G1, 30 (25%) to G2, and 35 (29.2%) to G3. Patients in G3 had worse renal function, functional class, and ejection fraction at the first visit and a more marked ejection fraction decline during follow-up, especially when compared with G1. Twenty-four patients (20%) died, 38 (31.7%) were admitted for heart failure, and 21 (17.5%) had atrial flutter/fibrillation during follow-up. These events were more frequent in G3 and significant differences were found compared with G1 in terms of death (HR, 2.9; 95%CI, 1.14-7.37; P=.026) and atrial flutter/fibrillation (HR, 2.9; 95%CI, 1.11-7.68; P=.037).
ConclusionsThe type of palliation in patients with SVP and restricted pulmonary flow not undergoing Fontan palliation identifies distinct profiles. Patients palliated with aortopulmonary shunts have an overall worse prognosis with higher morbidity and mortality.
Keywords
Single-ventricle physiology (SVP) represents 7.7% of congenital heart diseases diagnosed in childhood and has a birth prevalence of approximately 4-8 per 10 000 newborns.1 It is a rare and heterogeneous disorder that encompasses all forms of cardiac malformations that impede septation to a biventricular circulation.1 This mainly occurs when one of the ventricles is absent or hypoplastic and unable to sustain circulation. The other ventricle supports the volume overload from both pulmonary and systemic flow, establishing a parallel circulation.2
Depending on the anatomy, these patients often require a series of procedures early in life that provide initial palliation. Some patients will require an increase in pulmonary blood flow through a systemic to pulmonary shunt; others may benefit from a reduction in flow through banding of the pulmonary artery, while a few will manage without any intervention due to an advantageous anatomy.2 Nevertheless, the natural history of SVP patients with these initial palliation procedures is poor, with a reported mortality at 5 years of follow-up of 39%.3,4 However, the life expectancy of children born with SVP improved with the emergence of Fontan-type palliation.5 Survival in patients undergoing surgery after the year 2000 is> 95% at 10 years of follow-up.6
There are some patients who, for different reasons, are not suitable to complete the Fontan circulation and the initial palliation offered in infancy becomes definitive.7,8 There is limited information on the long-term prognosis of the rare cohort of patients with SVP and restrictive pulmonary blood flow who had not completed Fontan palliation; data are mostly limited to case reports or small case series and suggest that double inlet left ventricle with pulmonary stenosis may have a better outcome9 and that atrial flutter/fibrillation (AF/F) are more common in patients with aortopulmonary shunts.10
This study aimed to compare the survival and overall outcome of adult patients with SVP and restrictive pulmonary flow not completing the Fontan repair according to the type of definitive palliation. Differences in clinical, laboratory, and echocardiographic characteristics at baseline, at the end of follow-up, and cardiovascular events during follow-up are evaluated.
METHODSStudy design and patient populationThis multicenter retrospective observational study was performed with the participation of 7 adult congenital heart disease units in Spain. Patients with a diagnosis of SVP (namely, double inlet ventricle, mitral atresia/hypoplastic left heart syndrome, tricuspid atresia, pulmonary atresia with an intact ventricular septum, unbalanced atrioventricular septal defect, and complex double outlet right ventricle) were identified from the prospectively maintained databases of each participating unit. Patients with Fontan palliation and those with unrestricted pulmonary flow developing Eisenmenger physiology or segmental pulmonary hypertension were excluded. Patients were finally divided into 3 groups based on the type of definitive palliation; group 1 (G1): restrictive pulmonary forward flow, either by native pulmonary stenosis or by surgical banding, group 2 (G2): cavopulmonary shunt (CPS), and group 3 (G3): aortopulmonary shunts (±CPS).
Data collection and definition of outcomesThe study period was from May 1999 to March 2020. After identification of the study population, clinical charts were reviewed and baseline characteristics, recorded at the time of the first visit at the adult congenital heart disease unit were identified: age, sex, referral origin, underlying diagnosis, type of palliation, reason for not performing Fontan palliation, and prior events. Some variables were collected at the first and last follow-up visits: oxygen saturation; New York Heart Association (NYHA) functional class; anticoagulant treatment; 12-lead electrocardiogram; echocardiography parameters (ejection fraction (EF), atrioventricular and ventriculoarterial valvular function assessment); and variables from blood tests (hemoglobin concentration, iron concentration, glomerular filtration rate (GFR) and platelet count). During the follow-up, data were obtained from outpatient visits or hospital admissions. The primary endpoint was death, and the secondary endpoint was the combination of death, heart transplant, or admission for heart failure (HF). Surgical and percutaneous cardiac interventions and other events, such as sustained AF/F or sustained ventricular arrhythmias, implantable cardioverter-defibrillator or pacemaker implantation, stroke, major infection (requiring hospital admission), and endocarditis were also recorded.
Oxygen saturation was measured following a 5-minute resting period and electrocardiograms and echocardiograms were obtained during outpatient clinic appointments. Systolic ventricular function was estimated using the Simpson method. Systemic atrioventricular valve regurgitation was considered important if greater than or equal to moderate. We defined admission for HF when the admission was due to clinical signs and symptoms of heart failure (HF). SCD was defined as death within 1 hour of symptom onset or unwitnessed death during sleep. To ensure correct data completion, this study includes only those variables that were recorded in at least 75% of patients.
The study protocol was approved by the institutional review board at each institution. Individual consent was waived because of the retrospective and deidentified nature of the data.
Statistical analysisContinuous variables are reported as mean±standard deviation or median and 25th-75th percentiles. Categorical variables are reported as number (percentage). Based on the normality of the distribution (tested for normality using the Kolmogorov-Smirnov test), between-group comparisons of continuous variables was performed using variance and linear regression analyses. Categorical variables were compared using logistic regression.
The probability of survival and remaining free from an event after the first visit at the adult congenital heart disease unit was estimated by the Kaplan-Meier method and was compared among the 3 groups with the log-rank test. Univariate analysis of predictors of events was performed using the Cox proportional hazard model.
Changes in each variable during follow-up within each group were tested with the paired-samples t test for continuous variables and with the McNemar test for categorical variables. We also analyzed the effect of the group on the changes in each variable during follow-up adjusted to baseline using the ANCOVA test for continuous variables and logistic regression for categorical variables.
Three variables were dichotomized (GFR <60 mL/min/1.73 m2, platelet count <150 000 per mm3 and NYHA ≥ III). Cardiac physiology was considered a categorical variable. For all analyses, a 2-tailed P value <.05 was used as the criterion for statistical significance. All analyses were performed using STATA/IC software version 15.1 (College Station, USA).
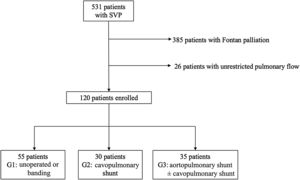
RESULTSA total of 513 patients with SVP were identified. After exclusion of individuals completing Fontan circulation and those with unrestricted pulmonary flow, we included 120 patients in the study. Patients were divided into 3 groups based on their initial palliation. Fifty five (45.8%) had not undergone surgery (n=41) or had their pulmonary forward flow restricted by pulmonary banding (n=14) (G1), 30 (25%) had undergone CPS (G2), and 35 (29.2%) had aortopulmonary shunts with (n=12) or without (n=23) CPS (G3) (figure 1).
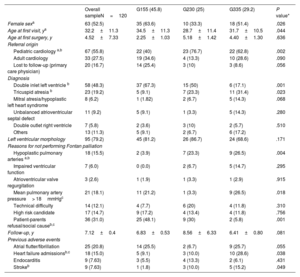
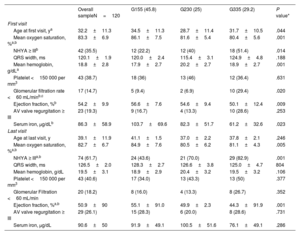
Baseline characteristicsThe baseline characteristics of the different groups are depicted in table 1. Mean age at first visit was 32.2±11.3 years and 63 (52.5%) were female. The morphologically left ventricle was more frequent (79.2%), with no differences between groups.
Baseline characteristics
| Overall sampleN=120 | G155 (45.8) | G230 (25) | G335 (29.2) | P value* | |
|---|---|---|---|---|---|
| Female sexa | 63 (52.5) | 35 (63.6) | 10 (33.3) | 18 (51.4) | .026 |
| Age at first visit, ya | 32.2±11.3 | 34.5±11.3 | 28.7±11.4 | 31.7±10.5 | .044 |
| Age at first surgery, y | 4.52±7.33 | 2.25±1.03 | 5.18±1.42 | 4.40±1.30 | .636 |
| Referral origin | |||||
| Pediatric cardiology a,b | 67 (55.8) | 22 (40) | 23 (76.7) | 22 (62.8) | .002 |
| Adult cardiology | 33 (27.5) | 19 (34.6) | 4 (13.3) | 10 (28.6) | .090 |
| Lost to follow-up (primary care physician) | 20 (16.7) | 14 (25.4) | 3 (10) | 3 (8.6) | .056 |
| Diagnosis | |||||
| Double inlet left ventricle b | 58 (48.3) | 37 (67.3) | 15 (50) | 6 (17.1) | .001 |
| Tricuspid atresia b | 23 (19.2) | 5 (9.1) | 7 (23.3) | 11 (31.4) | .023 |
| Mitral atresia/hypoplastic left heart syndrome | 8 (6.2) | 1 (1.82) | 2 (6.7) | 5 (14.3) | .068 |
| Unbalanced atrioventricular septal defect | 11 (9.2) | 5 (9.1) | 1 (3.3) | 5 (14.3) | .280 |
| Double outlet right ventricle | 7 (5.8) | 2 (3.6) | 3 (10) | 2 (5.7) | .510 |
| Others | 13 (11.3) | 5 (9.1) | 2 (6.7) | 6 (17.2) | |
| Left ventricular morphology | 95 (79.2) | 45 (81.2) | 26 (86.7) | 24 (68.6) | .171 |
| Reasons for not performing Fontan palliation | |||||
| Hypoplastic pulmonary arteries a,b | 18 (15.5) | 2 (3.9) | 7 (23.3) | 9 (26.5) | .004 |
| Impaired ventricular function | 7 (6.0) | 0 (0.0) | 2 (6.7) | 5 (14.7) | .295 |
| Atrioventricular valve regurgitation | 3 (2.6) | 1 (1.9) | 1 (3.3) | 1 (2.9) | .915 |
| Mean pulmonary artery pressure> 18mmHgc | 21 (18.1) | 11 (21.2) | 1 (3.3) | 9 (26.5) | .018 |
| Technical difficulty | 14 (12.1) | 4 (7.7) | 6 (20) | 4 (11.8) | .310 |
| High risk candidate | 17 (14.7) | 9 (17.2) | 4 (13.4) | 4 (11.8) | .756 |
| Patient-parents refusal/social causeb,c | 36 (31.0) | 25 (48.1) | 9 (30) | 2 (5.8) | .001 |
| Follow-up, y | 7.12±0.4 | 6.83±0.53 | 8.56±6.33 | 6.41±0.80 | .081 |
| Previous adverse events | |||||
| Atrial flutter/fibrillation | 25 (20.8) | 14 (25.5) | 2 (6.7) | 9 (25.7) | .055 |
| Heart failure admissionsb,c | 18 (15.0) | 5 (9.1) | 3 (10.0) | 10 (28.6) | .038 |
| Endocarditis | 9 (7.63) | 3 (5.5) | 4 (13.3) | 2 (6.1) | .431 |
| Strokeb | 9 (7.63) | 1 (1.8) | 3 (10.0) | 5 (15.2) | .049 |
G1, restrictive pulmonary forward flow; G2, cavopulmonary shunt (CPS), G3, aortopulmonary shunts (±CPS).
The data are presented as No. (%) or mean±standard deviation.
Patients in G1 were older at the first visit with significant differences compared with those in G2 (34.5±11.3 vs 28.7±11.4 years, P=.044) and were mostly referred from general cardiologists and primary care physicians (60%). The main underlying anatomy in this group was double inlet left ventricle. The main reasons for not undergoing Fontan-type palliation were parental refusal or social constraints (48.1%).
In contrast, patients in G2 and G3 were younger at the first visit (28.7±11.4 years and 31.7±10.5 years, respectively), were mostly referred from pediatric cardiology (76.7% and 62.8% respectively) and Fontan palliation was not performed mainly due to unfavorable anatomy or hemodynamics. The occurrence of adverse events showed significant differences: prior admission for HF was more frequent in G3 (P=.038), while stroke was less frequent in G1 (P=.049) (table 1).
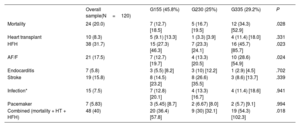
Adverse eventsDuring follow-up (7.1±0.4 years), 24 patients died, 10 received a heart transplant (2 died within the first 30 days after transplant), 38 were admitted for HF, and 21 had AF/F. Other relevant events recorded were 2 sustained ventricular arrhythmias, 7 endocarditis, 19 strokes, 15 major infections, and 7 pacemaker and 3 implantable cardioverter-defibrillator implants (table 2 and table 3). During follow-up, 27 patients needed surgical or percutaneous cardiac interventions, which were more frequent in G3 (). The most frequent cause of death was sudden cardiac death (SCD) (n=9, 38%) followed by HF (n=8, 33%), with no differences between groups.
Outcomes
| Overall sample(N=120) | G155 (45.8%) | G230 (25%) | G335 (29.2%) | P | |
|---|---|---|---|---|---|
| Mortality | 24 (20.0) | 7 (12.7) [18.5] | 5 (16.7) [19.5] | 12 (34.3) [52.9] | .028 |
| Heart transplant | 10 (8.3) | 5 (9.1) [13.3] | 1 (3.3) [3.9] | 4 (11.4) [18.0] | .331 |
| HFH | 38 (31.7) | 15 (27.3) [46.3] | 7 (23.3) [24.1] | 16 (45.7) [85.7] | .023 |
| AF/F | 21 (17.5) | 7 (12.7) [19.7] | 4 (13.3) [20.5] | 10 (28.6) [54.9] | .024 |
| Endocarditis | 7 (5.8) | 3 (5.5) [8.2] | 3 (10) [12.2] | 1 (2.9) [4.5] | .702 |
| Stroke | 19 (15.8) | 8 (14.5) [23.2] | 8 (26.6) [35.5] | 3 (8.6) [13.7] | .339 |
| Infection* | 15 (7.5) | 7 (12.8) [20.1] | 4 (13.3) [16.7] | 4 (11.4) [18.6] | .941 |
| Pacemaker | 7 (5.83) | 3 (5.45) [8.7] | 2 (6.67) [8.0] | 2 (5.7) [9.1] | .994 |
| Combined (mortality + HT + HFH) | 48 (40) | 20 (36.4) [57.8] | 9 (30) [32.1] | 19 (54.3) [102.3] | .018 |
AF/F, atrial flutter/fibrillation; G1, restrictive pulmonary forward flow; G2, cavopulmonary shunt (CPS); G3, aortopulmonary shunts (± CPS)
HFH, heart failure hospitalization; HT, heart transplant; IDR, incidence density rate.
The data are expressed as No. (%) or n, % [IDR 1000 person-year].
Univariate analysis
| G1 vs G2 | G1 vs G3 | G2 vs G3 | ||||
|---|---|---|---|---|---|---|
| HR(95%CI) | P | HR(95%CI) | P | HR(95%CI) | P | |
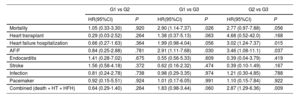
| Mortality | 1.05 (0.33-3.30) | .920 | 2.90 (1.14-7.37) | .026 | 2.77 (0.97-7.88) | .056 |
| Heart transplant | 0.29 (0.03-2.52) | .264 | 1.38 (0.37-5.13) | .063 | 4.68 (0.52-42.0) | .168 |
| Heart failure hospitalization | 0.66 (0.27-1.63) | .364 | 1.99 (0.98-4.04) | .056 | 3.02 (1.24-7.37) | .015 |
| AF/F | 0.84 (0.25-2.88) | .781 | 2.91 (1.11-7.68) | .030 | 3.46 (1.08-11.1) | .037 |
| Endocarditis | 1.41 (0.28-7.02) | .675 | 0.55 (0.56-5.33) | .609 | 0.39 (0.04-3.79) | .419 |
| Stroke | 1.56 (0.58-4.18) | .372 | 0.62 (0.16-2.32) | .474 | 0.39 (0.10-1.49) | .167 |
| Infection | 0.81 (0.24-2.78) | .738 | 0.98 (0.29-3.35) | .974 | 1.21 (0.30-4.85) | .788 |
| Pacemaker | 0.92 (0.15-5.51) | .924 | 1.01 (0.17-6.05) | .991 | 1.10 (0.15-7.84) | .922 |
| Combined (death + HT + HFH) | 0.64 (0.29-1.40) | .264 | 1.83 (0.98-3.44) | .060 | 2.87 (1.29-6.36) | .009 |
AF/F, atrial flutter/fibrillation; G1: restrictive pulmonary forward flow; G2: cavopulmonary shunt (CPS); G3: aortopulmonary shunts (± CPS); HFH, heart failure hospitalization; HT, heart transplant.
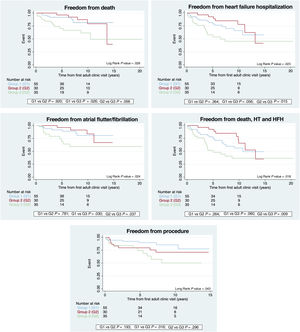
During follow-up, death was more frequent in G3, with significant differences compared with G1 (hazard ratio [HR], 2.9; 95% confidence interval [95%CI], 1.14-7.37; P=.026). Admissions for HF were also more frequent in G3, with significant differences compared with G2 (HR, 3.0; 95%CI, 1.24-7.37; P=.015). The incidence of AF/F was also more frequent in G3, when compared with both G1 (HR, 2.91; 95%CI, 1.11-7.68; P=.030) and G2 (HR, 3.46; 95%CI, 1.08-11.1; P=.037). G3 also required a larger number of surgical or percutaneous procedures, with significant differences compared with G1 (HR, 3.2; 95%CI, 1.24-8.28; P=.016). When we analyzed the combined endpoint (death, heart transplant, and admission for HF), G3 patients had worse outcomes with significant differences compared with G2 (HR, 2.87; 95%CI, 1.29-6.36; P=.009) (table 2 and table 3, figure 2).
Predictions of mortality, freedom from heart failure hospitalization, from atrial flutter/fibrillation and combined endpoint (death, HT, and HFH). Group 1 (G1): restrictive pulmonary forward flow; group 2 (G2): cavopulmonary shunt (CPS); group 3 (G3): aortopulmonary shunts (±CPS). HFH, heart failure hospitalization; HT, heart transplant.
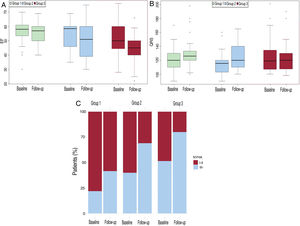
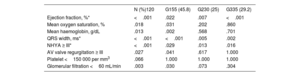
Table 4 shows the comparison of the clinical, blood test, and echocardiographic variables at the first and last visits. Patients in G1 had a better functional class, higher oxygen saturation at both time points, and, accordingly, lower mean hemoglobin levels (although differences in hemoglobin levels among groups at the end of follow-up were not statistically significant). Patients in G3 had lower ventricular EF, worse GFR, and lower serum iron levels at their first visit. The trend was maintained at the end of follow-up, although only EF was statistically significant.
Changes in baseline parameters during follow-up
| Overall sampleN=120 | G155 (45.8) | G230 (25) | G335 (29.2) | P value* | |
|---|---|---|---|---|---|
| First visit | |||||
| Age at first visit, ya | 32.2±11.3 | 34.5±11.3 | 28.7±11.4 | 31.7±10.5 | .044 |
| Mean oxygen saturation, %a,b | 83.3±6.9 | 86.1±7.5 | 81.6±5.4 | 80.4±5.6 | .001 |
| NHYA ≥ IIIb | 42 (35.5) | 12 (22.2) | 12 (40) | 18 (51.4) | .014 |
| QRS width, ms | 120.1±1.9 | 120.0±2.4 | 115.4±3.1 | 124.9±4.8 | .188 |
| Mean hemoglobin, g/dLa | 18.8±2.8 | 17.9±2.7 | 20.2±2.7 | 18.9±2.7 | .001 |
| Platelet <150 000 per mm3 | 43 (38.7) | 18 (36) | 13 (46) | 12 (36.4) | .631 |
| Glomerular filtration rate <60 mL/minb,c | 17 (14.7) | 5 (9.4) | 2 (6.9) | 10 (29.4) | .020 |
| Ejection fraction, %b | 54.2±9.9 | 56.6±7.6 | 54.6±9.4 | 50.1±12.4 | .009 |
| AV valve regurgitation ≥ III | 23 (19.3) | 9 (16.7) | 4 (13.3) | 10 (28.6) | .253 |
| Serum iron, μg/dLb | 86.3±58.9 | 103.7±69.6 | 82.3±51.7 | 61.2±32.6 | .023 |
| Last visit | |||||
| Age at last visit, y | 39.1±11.9 | 41.1±1.5 | 37.0±2.2 | 37.8±2.1 | .246 |
| Mean oxygen saturation, %a,b | 82.7±6.7 | 84.9±7.6 | 80.5±6.2 | 81.1±4.3 | .005 |
| NHYA ≥ IIIa,b | 74 (61.7) | 24 (43.6) | 21 (70.0) | 29 (82.9) | .001 |
| QRS width, ms | 126.5±2.0 | 128.3±2.7 | 126.6±3.8 | 125.0±4.7 | 804 |
| Mean hemoglobin, g/dL | 19.5±3.1 | 18.9±2.9 | 20.4±3.2 | 19.5±3.2 | .106 |
| Platelet <150 000 per mm3 | 43 (40.6) | 17 (34.0) | 13 (43.3) | 13 (50) | .377 |
| Glomerular Filtration <60 mL/min | 20 (18.2) | 8 (16.0) | 4 (13.3) | 8 (26.7) | .352 |
| Ejection fraction, %a,b | 50.9±90 | 55.1±91.0 | 49.9±2.3 | 44.3±91.9 | .001 |
| AV valve regurgitation ≥ III | 29 (26.1) | 15 (28.3) | 6 (20.0) | 8 (28.6) | .731 |
| Serum iron, μg/dL | 90.6±50 | 91.9±49.1 | 100.5±51.6 | 76.1±49.1 | .286 |
AV, atrioventricular; G1, restrictive pulmonary forward flow; G2, cavopulmonary shunt (CPS); G3, aortopulmonary shunts (±CPS), NHYA, New York Heart Association functional classification.
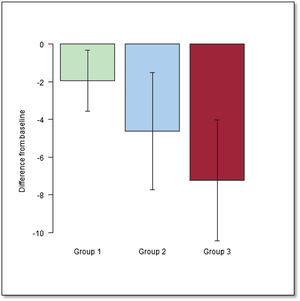
We also analyzed the change in baseline variables during the study period within each group. In all 3 groups, EF, QRS width, and functional class deteriorated over time (P <.001) (table 5 and figure 3). We also assessed the effect of the type of palliation in these changes. After adjustment to the initial value, the decline in EF was significantly worse when we compared G1 and G3 (P <.001) (figure 4 and figure 5).
Significance (P value) for comparison between baseline and follow-up values for each variable overall and in each group
| N (%)120 | G155 (45.8) | G230 (25) | G335 (29.2) | |
|---|---|---|---|---|
| Ejection fraction, %* | <.001 | .022 | .007 | <.001 |
| Mean oxygen saturation, % | .018 | .031 | .202 | .860 |
| Mean haemoglobin, g/dL | .013 | .002 | .568 | .701 |
| QRS width, ms* | <.001 | <.001 | .005 | .002 |
| NHYA ≥ III* | <.001 | .029 | .013 | .016 |
| AV valve regurgitation ≥ III | .027 | .041 | .617 | 1.000 |
| Platelet <150 000 per mm3 | .066 | 1.000 | 1.000 | 1.000 |
| Glomerular filtration <60 mL/min | .003 | .030 | .073 | .304 |
AV, atrioventricular; NHYA, New York Heart Association functional classification.
To the best of our knowledge, this is the first study to comprehensively characterize and compare the different populations of patients with univentricular hearts with restricted pulmonary flow surviving into adulthood without completing Fontan-type palliation. According to our findings, patients requiring aortopulmonary shunts (G3) faced the worse scenario, with worse functional class, ventricular, and renal function at the first visit, a more marked decline in EF during follow-up, and a higher risk of adverse events, namely overall mortality, admissions for HF, AF/F, and need for procedures during follow-up. At the other end of the spectrum, patients with well-balanced restricted pulmonary forward flow, either via native pulmonary stenosis or pulmonary banding (G1) had the best clinical scenario, with better oxygen saturation and functional class at the first visit and during follow-up. Patients palliated with a CPS (G2) were in an intermediate situation in terms of their baseline characteristics and follow-up course.
Information on patients with functionally univentricular hearts not completing Fontan circulation is scarce and the published studies are widely heterogeneous in the definition of patient subpopulations, length of follow-up, and type of outcomes reported. Gatzoulis et al.,10 excluded patients with pulmonary hypertension and divided their cohort into 2 groups: those palliated with aortopulmonary shunts only and those with CPS with or without additional aortopulmonary shunts. On the other hand, the cohort of Poterucha J et al.,9 was limited to unoperated patients surviving more than 3 decades and was divided into patients with native pulmonary stenosis and with Eisenmenger syndrome. The purpose of our study was to comprehensively characterize and compare univentricular patients, dividing them into subgroups with different and characteristic pathophysiology. We excluded patients with pulmonary hypertension (Eisenmenger syndrome and segmental pulmonary hypertension) since, pathophysiologically, they represent a group with already well-known and well-defined characteristics.11 In addition, we separately analyzed those patients with aortopulmonary shunts since, to date, they have shown the greatest pathophysiological and prognostic differentiation.10,12
In our population, G1 (restricted pulmonary forward flow) had the most favorable profile, with the best functional class, oxygen saturation, and EF at the beginning and end of the follow-up. Patients in this group were older at the first visit to the adult congenital heart disease unit (34.5±11.3 years) and had lower mortality rates (12.7%). Several reasons could explain their overall favorable profile: a) more than 75% had a native balanced circulation that had not required any type of surgery during their life; b) nearly 64% had an underlying diagnosis of double inlet left ventricle, a condition that has been reported to have higher survival rates in previously published series9,13,14; c) the main reason for not performing Fontan palliation was not related to a worse anatomic or hemodynamic profile but rather to patient-parental refusal/social constraints (48.1%); and d) this group possibly had a greater immortality bias with older age at referral due to this favorable anatomy.
At the other end of the spectrum, a systolic and diastolic (as opposed to exclusively systolic in G1) volume overload imposed by aortopulmonary shunts15,16 may partially explain the overall worse status and prognosis of patients in G3. In addition, a more marked cyanosis, with its consequent impact on long-term myocardial performance, may account for the requirement of this additional source of pulmonary flow in this cohort. These patients had worse EF at the first visit (EF 50.1±12.4%) and more marked deterioration over time, a finding that had been previously reported. Gatzoulis et al.,10 also described a higher rate of arrhythmias in this population, which can be considered another indicator of marked overload, and which was also found in our series (28.6% atrial arrhythmias). In line with this, HF admissions were higher in this group (45%), as were the mortality rate (34.3%) and the combined event of mortality, heart transplant, or admission for HF (54.3%).
Patients in G2 (CPS) had an intermediate profile between the other 2 groups in terms of functional class, EF, mortality, and AF/F. Vermuat et al.17 reported worse outcomes in patients with CPS as the definitive palliation. After 11 years of follow-up, the rates were 20% for mortality, 40% for HF, and 20% for atrial fibrillation, whereas in our series, after a follow-up of 7.12±0.4 years, we found rates of 16.7% for mortality, 23.3% for HF admission, and 13.3% for atrial arrhythmias. In addition to the length of follow-up, the main difference between this series and our own is that aortopulmonary shunts were excluded from our CPS group, a finding that underscores the importance of volume overload of the single ventricle as a determinant of long-term prognosis.
In our series, the main cause of death was SCD (38%), closely followed by HF (33%), with no significant differences between groups. This finding is in agreement with previous late outcome reports in univentricular patients without Fontan palliation9,10 and an isolated series of cavopulmonary17,18 and aortopulmonary shunts.19 Nevertheless, we observed a low incidence of sustained ventricular arrhythmias, suggesting that other causes are implicated in the etiology of SCD. For example, atrial arrhythmias (we observed 17.5% of AF/F) could be poorly tolerated hemodynamically or could be a trigger for ventricular arrhythmias. Moreover, bradyarrhythmia and advanced atrioventricular block (5.8% had a pacemaker) could also be involved. These data are important since the selection of patients for primary prevention is a challenge in this population, as evidenced by the low rate of implantable cardioverter-defibrillator implantation.
Interestingly, no differences between groups were detected when we analyzed thrombocytopenia (platelets <150 000 per mm3), QRS width, and atrioventricular valve failure; these 3 variables have been found to be independent predictors of mortality in our previously published series of SVP, which included patients with pulmonary hypertension.20
LimitationsGiven the retrospective observational nature of our study, our findings remain susceptible to unmeasured confounders. Due to the absence of a prospective data collection plan, some important variables have not been analyzed. Another limitation is that, despite being a multicenter study, the sample size remained small. Furthermore, this is a very heterogeneous population referred from different centers with no standardized selection criteria for the type of palliation. However, no significant differences were detected among centers in the type of palliation performed.
Regarding methods, the Simpson method was used to assess EF with the intrinsic limitation of the method in this type of patient, particularly in morphologically right ventricles.
On the one hand, and due to immortal bias, only patients surviving to adulthood could be included in the study and, consequently, may represent the best profile on the spectrum of this type of patient. This could be especially relevant in G1, the group with the best clinical profile, containing up to 60% of the late referrals from community cardiologists or general practitioners. However, when we compared baseline characteristics and follow-up events of patients referred from pediatric care to those from the community in G1, no relevant differences were found (except baseline EF: 54% vs 58%, P=.048, favoring patients from the community). On the other hand, and due to referral bias, sicker patients might have been more likely to be included since only tertiary referral centers participated in patient enrolment.
CONCLUSIONSIn the cohort of patients with SVP and restrictive pulmonary flow not completing Fontan circulation, the type of definitive palliation defined very differentiated patient profiles. Patients requiring aortopulmonary shunts had the least favorable scenario, with worse functional class, ventricular and renal function at the first visit, a more marked decline in ventricular function during follow-up, and a higher risk of adverse events, namely, overall mortality, admissions for HF, atrial arrhythmias, and requirement for procedures over time. At the other end of the spectrum, patients with well-balanced restricted pulmonary forward flow had the best clinical situation. Finally, patients palliated with CPS were in an intermediate position, both in terms of baseline characteristics and follow-up course.
- -
It is known that patients with SVP who cannot achieve biventricular correction have a better prognosis if they undergo Fontan palliation. However, not all patients have been able to benefit from Fontan surgery or are suitable candidates for the procedure, as they are unpalliated or have initial/intermediate palliations. The outcome of these patients according to the type of palliation received is unknown.
- -
We analyzed the largest published series of patients with SVP and restrictive pulmonary flow not undergoing Fontan circulation completion. We divided the patients into 3 groups according to the definitive palliation performed and found that patients requiring aortopulmonary shunts had a worse scenario and those with well-balanced restricted pulmonary forward flow had the best clinical situation.
No funding.
AUTHORS’ CONTRIBUTIONSAll authors have contributed to data collection, data analysis, and manuscript drafting and revision.
CONFLICTS OF INTERESTNone declared.
Our sincere thanks to José M. Oliver who has taught us so much and who unfortunately passed away suddenly during the writing of this manuscript.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2023.03.001