This article provides a detailed review of the most important studies on interventional cardiology reported in publications or presentations during the year 2012. With regard to coronary interventions, ST-elevation myocardial infarction is extensively addressed in studies focusing on the relevance of reducing the reperfusion time and the utility of various devices and pharmacological strategies in primary angioplasty. Multiple comparative studies involving different generations of drug-eluting stents are available and indicate a favorable progression in terms of safety and efficacy. The risk of late thrombosis with the new generations of drug-eluting stents seems to be equivalent to that observed with bare-metal stents. The clinical outcomes with these stents in the elderly, in left main coronary artery, or in multivessel disease have also been the subject of important trials. Among the studies on intracoronary diagnostic techniques, those correlating imaging and pressure-based techniques are of special interest. The percutaneous treatment of structural heart disease, particularly transcatheter aortic valve implantation, followed by mitral repair, continues to be the subject of a great number of publications. Finally, renal denervation is currently being widely discussed in the literature.
Keywords
.
CLINICAL SITUATIONSST-segment Elevation Myocardial Infarction: Implementation of Primary Percutaneous Coronary InterventionThe importance of delays in reperfusion by means of primary percutaneous coronary intervention (PCI) has again been well established in a registry of 107028 patients treated with fibrinolysis or transferred to another center for PCI.1 It was observed that a delay of over 120min resulting from patient transfer negated the survival benefit. The PROGALIAM group, a pioneer in Spain in the design of these network programs, demonstrated that their application has made it possible to increase the proportion of patients treated with primary angioplasty, while maintaining the results of this therapy.2
StentsWith respect to the devices employed in PCI, specifically the drug-eluting stents (DES), Hofma et al. have published the results of the XAMI trial, which compared everolimus-eluting stents (EES) with sirolimus-eluting stents (SES), showing that the former were not inferior to the latter and were even associated with a trend toward better outcomes.3
The Spanish ESTROFA-MI registry compared EES and paclitaxel-eluting stents in 734 patients. The investigators observed a lower incidence of thrombosis and myocardial infarction and a strong trend toward a reduced need for revascularization with EES.4
Other devices used in this context are the paclitaxel-eluting balloons. In the DEB-AMI trial, a randomized study, the use of these balloons together with bare-metal stents (BMS) was associated with a higher rate of restenosis than that observed with DES and an incidence similar to that resulting from the use of BMS alone.5
Vascular AccessVascular access was evaluated in the RIFLE study, a large trial in which 1001 patients with acute coronary syndrome were randomized to radial or femoral access.6 The use of the radial approach was associated with a lower 30-day incidence of adverse events (13.6% vs 21%; P=.003).
Multivessel DiseaseThe optimal approach in patients with acute myocardial infarction and multivessel disease was the subject of an extensive meta-analysis. The results demonstrated that multivessel revascularization performed during the primary PCI procedure had adverse effects and that, in contrast, deferred intervention in the nonculprit vessel lesions produced favorable outcomes.7
Support in Primary Percutaneous Coronary InterventionsWith respect to concomitant drug therapy, the AIDA STEMI trial, involving 2065 patients, did not demonstrate that intracoronary versus intravenous abciximab reduced the primary endpoint of death, myocardial infarction, and heart failure at 90 days (7% vs 7.6%), but intracoronary administration was associated with a lower incidence of heart failure (2.4% vs 4.1%; P=.04); thus, given that it is a safe approach, its use can be recommended.8
Thrombectomy devices and the intracoronary administration of abciximab with the ClearWay® site-specific microcatheter were the topics of a randomized factorial trial (INFUSE AMI), in which the primary endpoint was the size of the myocardial infarction after 30 days, measured by magnetic resonance imaging.9 No benefit was observed with thrombectomy, but there was an evident benefit with intracoronary abciximab (an absolute reduction of 2.8%), especially when combined with thrombectomy.
Acute Coronary Syndrome Without ST Segment ElevationWith respect to antiplatelet therapy, a study of 302 patients who received a loading dose of prasugrel found that a fourth of them had suboptimal platelet inhibition 6h to 12h later, a circumstance that was related to an increase in clinical events.10
A substudy of the PLATO trial with ticagrelor showed that the benefit in the patients enrolled in North America was inferior to that observed in the rest of the world, a difference that could be explained, at least in part, by the fact that the North American patients received higher doses of acetylsalicylic acid, which reduced the positive impact of the drug.11
With regard to anticoagulants, in the ATLAS trial involving more than 15 000 patients with acute coronary syndrome, the dose of rivaroxaban of 2.5mg twice daily reduced the 13-month mortality with respect to placebo and the 5-mg dose, but there was a significant increase in major bleeding and intracranial hemorrhages, with no increase in fatal hemorrhages.12 A study with apixaban was interrupted prematurely due to a significant increase in bleeding, with no benefit in terms of ischemic events.13
Elderly PatientsThe performance of PCI in acute coronary syndrome without ST-segment elevation in octogenarian patients was the subject of a study carried out by a Spanish group that observed clinical benefits in its application.14 The majority of the patients had a high-risk profile and, after propensity score matching, revascularization was found to be associated with reductions in the composite of death, myocardial infarction, and major adverse cardiac events.
Diabetic PatientsSurgery was compared with DES in a Korean registry of 891 diabetic patients with multivessel disease who were followed for 5 years. After statistical adjustments, no significant differences were observed between the 2 approaches in terms of death, myocardial infarction, or stroke, but revascularization was performed more frequently with DES.15
TYPES OF CORONARY LESIONSLeft Main Coronary ArteryThe results of a second phase of the PRECOMBAT trial, which compared surgery with SES, have been published. In the PRECOMBAT-2 trial, these 2 groups were compared with a group treated with EES. The patients who received the EES had an overall rate of events at 18 months comparable to those recorded for SES and surgery.16 The need for revascularization was less frequent with surgery and was similar with both DES. A large international registry (DELTA), with 2775 patients, compared surgery and DES and, after 3 years of follow-up, found differences only in the need for repeat revascularization, which was less frequent following surgery.17
The results of 2 Spanish multicenter studies were also reported. In the first, the authors studied 226 patients who were not candidates for surgery.18 There was a high incidence of events during follow-up; the adverse predictors were female sex, ventricular dysfunction, and the use of BMS. The other study was the ESTROFA-LM registry,19 which compared PES with EES in 770 patients. No clinically significant differences were found between the stents, even after propensity score matching. The use of 2 stents in distal lesions proved to be an adverse predictor.
Finally, a meta-analysis of 4 available trials revealed no differences between DES and surgery in terms of total events at 1 year (14.5% vs 11.8%; P=.1), although the need for revascularization was less frequent with surgery and the incidence of stroke was lower with DES, whereas the rates of mortality and myocardial infarction were similar.20
Multivessel DiseaseA Korean registry, with more than 3000 patients and a follow-up period of 5 years, detected similar rates of death and a greater need for revascularization with DES than with surgery.21
Chronic OcclusionsThe rate of successful revascularization of chronic lesions has increased in recent years. Two studies have demonstrated that successfully treated patients have a lower rate of major cardiac events than those in whom the attempt is unsuccessful.22,23 A Spanish study has investigated the determining factors of successful recanalization using computed tomography; the authors report that an arc of calcification affecting more than 50% of the proximal and middle thirds of the occluded segment of the vessel was the only predictor of failure.24
The CIBELES study, with 207 randomized patients in Spain and Portugal, compared EES and SES in the treatment of chronic occlusions. The results show the noninferiority of the former with respect to the angiographic objective, with a comparable clinical course. Nevertheless, there was a notable trend toward less thrombosis with EES.25
Bifurcation LesionsA randomized study by the Spanish CORPAL group compared EES and SES in patients treated with a single stent in the main vessel. The investigators found no differences in the incidence of clinical events (death, myocardial infarction, and revascularization) at 1 year.26
Saphenous VeinsRecently, Mehilli et al. published the findings of a randomized study involving angioplasty in saphenous vein grafts with first-generation DES vs BMS in 610 patients. The results revealed a significant reduction in the primary endpoint (death, myocardial infarction, and target lesion revascularization in a group of patients treated with DES, mainly due to a reduction in target lesion revascularization.27
RestenosisIn a Korean study, 96 patients with focal restenosis of a DES were randomized to DES or cutting balloon angioplasty, and 66 with diffuse restenosis to SES or EES. SES were superior in the treatment of focal lesions, whereas the results with the 2 stents in diffuse lesions were comparable.28
The Spanish RIBS-3 study evaluated the treatment of restenosis of a DES using a different DES vs other alternatives.29 With a median follow-up of somewhat over 2 years, treatment with another DES proved to be superior (death, myocardial infarction, and target lesion revascularization, 23% vs 35%; P=.039).
DRUG-ELUTING STENTSSecond-generation Drug-eluting StentsThe RESET trial included 3197 patients randomized to EES or SES.30 The rate of target lesion revascularization, the primary endpoint, at 1 year was very similar (4.3% with EES and 5% with SES), as was that of definite stent thrombosis (0.32% and 0.38%, respectively).
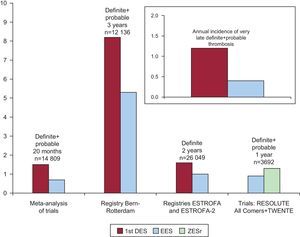
A number of studies, meta-analyses, and reviews have coincided in demonstrating a lower incidence of definite or probable stent thrombosis with EES when compared with first-generation DES, especially those delivering paclitaxel.31–36 In a large online meta-analysis, after 2 years EES showed a lower risk of thrombosis than other DES and even lower than that associated with BMS (Fig. 1).36
Comparison of the rates of thrombosis in first and second-generation drug-eluting stents. 1st DES, first-generation drug-eluting stents; EES, everolimus-eluting stent; ZESr, RESOLUTE zotarolimus-eluting stents. Reproduced with permission from de la Torre Hernández and Windecker35.
The TWENTE trial employed zotarolimus-eluting stents (Resolute®) in 1391 unselected patients (all comers), comparing them with EES; the investigators established the noninferiority of the former versus the latter.37 The RESOLUTE US trial produced excellent results in 1402 patients treated with the Resolute® stent, with a 1-year thrombosis rate of 0.1% and a target lesion revascularization rate of 2.8%.38
Biodegradable Polymer-coated Drug-eluting StentsAfter a 4-year follow-up period, the LEADERS trial reflected a lower incidence of thrombosis with biodegradable polymer biolimus-eluting stents than with durable polymer SES.39 A meta-analysis of the ISAR-TEST 3, ISAR-TEST 4, and LEADERS trials detected a lower risk of thrombosis at 4 years with biodegradable polymer DES than with those coated with a durable polymer, especially in the late phase.40
A novel bioabsorbable polymer DES (Synergy®), with the everolimus coating on the abluminal surface, was evaluated in the EVOLVE trial, with a lumen loss at 6 months of 0.1mm (0.13mm with the half dose).41
Fully Biodegradable Drug-eluting StentsAfter 12 months, the second generation of bioabsorbable EES showed a lumen loss of 0.27mm, with no loss in the vascular scaffold area and with coating of 97% of the struts, which had dissolved completely at 2-year follow-up.42
THE DRUG-ELUTING BALLOONThe PEPCAD-DES trial randomized 110 patients with restenosis after DES placement to paclitaxel-coated balloon angioplasty or to plain balloon angioplasty, demonstrating the superiority of the former, with 17.2% and 58.1% of restenosis, respectively (P=.001).43
STENT THROMBOSIS AND ANTIPLATELET THERAPYA collaborative study of 30 trials involving 221066 patients showed that the most important risk factors of stent thrombosis are the early interruption of antiplatelet therapy, the extent of coronary artery disease, and the number and length of the stents.44 The TRIGGER PCI trial has demonstrated that the use of prasugrel in patients with high platelet reactivity being treated with clopidogrel makes it possible to achieve an adequate level of platelet inhibition.45 Given the very small sample size, it was not possible to demonstrate clinical benefit with this strategy.
With respect to the duration of dual antiplatelet therapy, in the RESET trial, 2117 patients were randomized to 3 months of dual therapy with zotarolimus-eluting stents (Endeavor®) or standard therapy with other second-generation DES. The study established the noninferiority of the 3-month therapy, with thrombosis at 1 year of 0.2% (vs 0.3% in the standard group).46
INTRACORONARY DIAGNOSTIC TECHNIQUESIntracoronary ImagingThe left main coronary artery continues to be the subject of studies to define the parameters of coronary stenosis showing statistical significance in intravascular ultrasound. A Korean study proposed that a lumen area of 4.8mm2 correlated well with the fractional flow reserve (FFR).47 However, the study is small (55 patients) and has no prospective clinical validation, as the authors did not adhere to the protocol (30% of the patients with FFR greater than 0.8 underwent revascularization) and no data on the clinical follow-up is reported. Moreover, it is known that population differences (racial) can result in distinct cutoff points, given the differences in body size. In the left main coronary artery, the aforementioned ESTROFA-LM registry reported a positive impact with the use of intravascular ultrasound in PCI in distal lesions.19
A Spanish study of notable quality evaluated a series of 61 intermediate lesions using the 3 techniques—intravascular ultrasound, optical coherence tomography, and FFR.48 Although optical coherence tomography showed a greater diagnostic efficiency than intravascular ultrasound for identifying a lesion with FFR<0.8, its utility for defining the severity of the lesion is also very limited by its low specificity. The lumen area that was predictive of FFR<0.8 with this technique was 2mm2.
Pressure WireA Spanish study demonstrated that FFR is highly useful in the evaluation of nonculprit lesions in patients with acute coronary syndrome. After 1 year, only 3.7% of the untreated lesions required PCI.49
The FAME II study randomized patients with stable angina and at least 1 lesion with FFR<0.8 to PCI plus optimal medical treatment or to medical treatment alone.50 An excess of events in the group receiving only medical treatment made it necessary to terminate the study before planned. These results help to explain the lack of clinical benefit observed with PCI in trials like COURAGE, studies that most likely included a considerable proportion of patients with angiographically significant lesions (stenosis>50%), but with no flow disturbance (FFR>0.8).
The acquisition by means of pressure wire of the instantaneous wave-free ratio is a very novel technique that has an excellent correlation with the FFR and could be an alternative to it that, moreover, does not require adenosine infusion.51
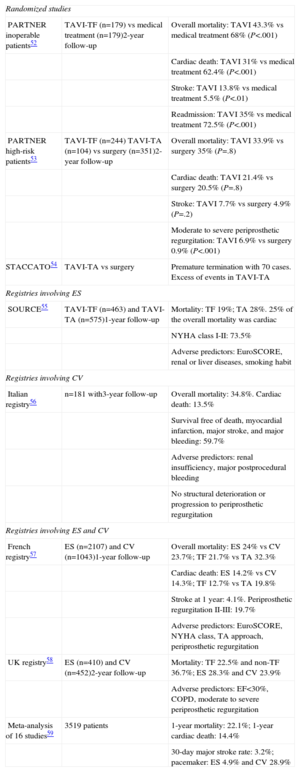
STRUCTURAL CARDIAC INTERVENTIONAL PROCEDURESTranscatheter Aortic Valve ImplantationThe 2-year outcomes of the 2 parts of the PARTNER trial, one dealing with inoperable patients and the other involving patients with high surgical risk,52,53 were published in 2012. The results of a number of registries were also published between 2011 and 2012. Among them, the most noteworthy studies were the French registry, because of its large size, and an extensive meta-analysis.54–59 The major findings of these studies are summarized in the Table.
Studies on Transcatheter Implantation of Aortic Valve Prostheses
| Randomized studies | ||
| PARTNER inoperable patients52 | TAVI-TF (n=179) vs medical treatment (n=179)2-year follow-up | Overall mortality: TAVI 43.3% vs medical treatment 68% (P<.001) |
| Cardiac death: TAVI 31% vs medical treatment 62.4% (P<.001) | ||
| Stroke: TAVI 13.8% vs medical treatment 5.5% (P<.01) | ||
| Readmission: TAVI 35% vs medical treatment 72.5% (P<.001) | ||
| PARTNER high-risk patients53 | TAVI-TF (n=244) TAVI-TA (n=104) vs surgery (n=351)2-year follow-up | Overall mortality: TAVI 33.9% vs surgery 35% (P=.8) |
| Cardiac death: TAVI 21.4% vs surgery 20.5% (P=.8) | ||
| Stroke: TAVI 7.7% vs surgery 4.9% (P=.2) | ||
| Moderate to severe periprosthetic regurgitation: TAVI 6.9% vs surgery 0.9% (P<.001) | ||
| STACCATO54 | TAVI-TA vs surgery | Premature termination with 70 cases. Excess of events in TAVI-TA |
| Registries involving ES | ||
| SOURCE55 | TAVI-TF (n=463) and TAVI-TA (n=575)1-year follow-up | Mortality: TF 19%; TA 28%. 25% of the overall mortality was cardiac |
| NYHA class I-II: 73.5% | ||
| Adverse predictors: EuroSCORE, renal or liver diseases, smoking habit | ||
| Registries involving CV | ||
| Italian registry56 | n=181 with3-year follow-up | Overall mortality: 34.8%. Cardiac death: 13.5% |
| Survival free of death, myocardial infarction, major stroke, and major bleeding: 59.7% | ||
| Adverse predictors: renal insufficiency, major postprocedural bleeding | ||
| No structural deterioration or progression to periprosthetic regurgitation | ||
| Registries involving ES and CV | ||
| French registry57 | ES (n=2107) and CV (n=1043)1-year follow-up | Overall mortality: ES 24% vs CV 23.7%; TF 21.7% vs TA 32.3% |
| Cardiac death: ES 14.2% vs CV 14.3%; TF 12.7% vs TA 19.8% | ||
| Stroke at 1 year: 4.1%. Periprosthetic regurgitation II-III: 19.7% | ||
| Adverse predictors: EuroSCORE, NYHA class, TA approach, periprosthetic regurgitation | ||
| UK registry58 | ES (n=410) and CV (n=452)2-year follow-up | Mortality: TF 22.5% and non-TF 36.7%; ES 28.3% and CV 23.9% |
| Adverse predictors: EF<30%, COPD, moderate to severe periprosthetic regurgitation | ||
| Meta-analysis of 16 studies59 | 3519 patients | 1-year mortality: 22.1%; 1-year cardiac death: 14.4% |
| 30-day major stroke rate: 3.2%; pacemaker: ES 4.9% and CV 28.9% | ||
COPD, chronic obstructive pulmonary disease; CV, CoreValve®; EF, ejection fraction; ES: Edwards-SAPIEN®; NYHA, New York Heart Association; TA, transapical; TAVI, transcatheter aortic valve implantation; TF, transfemoral.
The most notable aspects are:
- •
The comparable rate of mortality associated with the devices.
- •
The higher mortality rate with the transapical approach (related in part to a higher baseline risk).
- •
A considerable proportion of the medium- to long-term mortality is noncardiac.
- •
The 1-year stroke rate is 4% to 6%.
- •
The adverse prognostic effect of periprosthetic regurgitation, which is moderate or severe in 7% to 20% of patients at 1-year follow-up.
Two substudies of the EVEREST II trial evaluated the results in patients with atrial fibrillation and in those at high surgical risk.63,64 The benefit of this technique in terms of clinical course, as well as echocardiographic improvement, was observed in both subgroups.
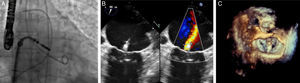
The PERMIT-CARE trial dealt with nonresponders to cardiac resynchronization therapy. Following mitral repair, an improvement was observed in the patients’ functional class, with reduced ventricular volumes and increased ventricular function.65Figure 2 shows different images of the MitraClip® implantation procedure.
Patent Foramen OvaleA meta-analysis on transcatheter closure versus medical therapy for the prevention of events due to presumed paradoxical embolism in patients with previous stroke has recently been published. Several closure devices were dealt with. The incidence of neurological events was 0.8 per 100 patient-years following transcatheter closure and 5 per 100 patient-years with medical treatment.66
Atrial Septal DefectsIn a review of the published reports of erosion of the aorta and left atrium associated with percutaneous closure devices, Crawford et al. found 104 cases involving the Amplatzer® occluder (estimated rate of 0.1% to 0.3%), related to oversized devices and deficient retroaortic rim.67
The REPERA registry retrospectively reviews 450 patients with septal defects treated with large occlusive devices (>26 mm) in 20 hospitals in Spain and Portugal.68 The procedure was successful in 92% of the patients; implantation was not possible in 4.4% and embolization occurred within the first 24h in 3.6%. During follow-up, the rate of serious complications was 1.8% (embolization, 0.8%; malposition, 0.6%; perforation, 0.4%). A deficient lower rim and oversized device (≥38 mm) were the predictors of failure.
Left Atrial AppendageA subanalysis of the PROTECT AF study has assessed the frequency and clinical impact of incomplete left atrial appendage closure following percutaneous device implantation. Transesophageal echocardiography demonstrated that 32% of the patients had some degree of leakage 12 months after implantation, but this was not associated with an increase in the risk of symptomatic embolism.69
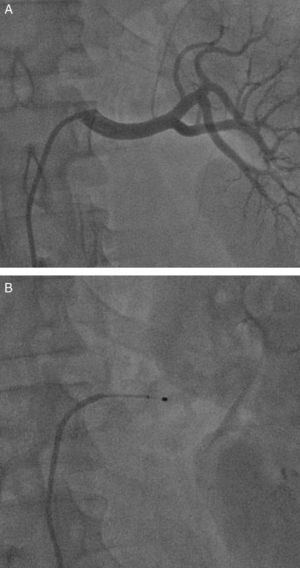
RENAL DENERVATIONIn a study of 50 patients (37 treated and 13 controls, with a follow-up period of 3 months), in addition to a reduction in arterial blood pressure, the authors found decreases in fasting glucose, in insulin, and in C peptide.70 In another study involving 64 patients (46 treated and 18 controls, with a follow-up period of 6 months), a reduction in hypertrophy and improved ventricular function were observed.71Figure 3 shows 2 aspects of the renal denervation procedure.
CELL THERAPYThe results of the FOCUS CCTRN trial were presented at the 2012 meeting of the American College of Cardiology.72 The study randomized 92 patients with ventricular dysfunction due to chronic ischemic heart failure to transendocardial injection of mononuclear cells or to placebo. Six months later, there was no evidence of changes in myocardial function or perfusion, or in the clinical situation.
A meta-analysis of 50 studies found that bone marrow cell therapy in adults produces long-term improvement in the ejection fraction in 4% of the patients and a reduction in ventricular volumes.73 Improvements in the rates of mortality and myocardial infarction were also observed.
CONFLICTS OF INTERESTNone declared.