In the era of transcatheter aortic valve implantation, sutureless Perceval valves (Livanova, London, United Kingdom) have emerged as a new implantation technique to minimize surgical risk in older patients with multiple comorbidities. This bioprosthesis facilitates a minimally invasive approach, allowing shorter duration of cross-clamping,1 and prevents patient-prosthesis mismatch because of the absence of a sewing ring, which allows a larger effective orifice area (EOA).2,3 As a result, sutureless valves have recently been proposed as an ideal solution for elderly patients with a small annulus.
Like other bioprostheses, sutureless valves degenerate; however, stent infolding with distortion (or “stent creep”) has been described as a single mechanism of valve failure. It consists of an inward deflection of the stent posts that leads to a reduced EOA, causing high gradients or paravalvular leaks.
Valve-in-valve (ViV) therapy using transcatheter heart valves (THVs) has been shown to be safe and effective in most patients with degenerated prosthetic valves. Nevertheless, little is known about ViV within sutureless valves and only a few cases have been reported.1,3 We present 5 illustrative cases of sutureless Perceval valve failure occurring at a median time of 3 years after the surgical aortic valve replacement and which were treated between December 2018 and December 2019.
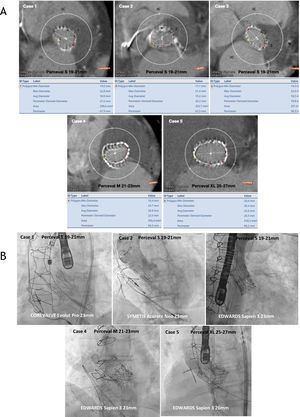
Baseline and procedural characteristics are outlined in table 1. Most of the patients were female and at high risk of aortic valve redo surgery (logistic EuroSCORE=38.7%, EuroSCORE II=24.3, and Society of Thoracic Surgeons score=13.4). The most frequent mechanism of sutureless bioprosthetic failure in our series was stenosis (n=2), regurgitation (n=1), and mixed (n=2). The mechanisms were stent infolding in 2 patients (figure 1), severe aortic regurgitation without evidence of endocarditis on positron emission-computed tomography in 1 patient, and elevated gradients due to calcification and valve degeneration in the other patients. Three of the treated valves were Perceval S (19-21mm), 1 was Perceval M (21-23mm), and 1 was Perceval XL (23-25mm).
Baseline and procedural characteristics and results
| Baseline characteristics | n=5 |
| Age, y | 83±3.43 |
| Female sex | 4 (80) |
| Hypertension | 5 (100) |
| Diabetes | 2 (40) |
| Dyslipidemia | 5 (100) |
| BMI, kg/m2 | 29.18 |
| Logistic EuroSCORE, % | 38.67 |
| Euroscore II | 24.27 |
| STS score | 13.38 |
| NYHA class IV | 4 (80) |
| Coronary artery disease | 2 (40) |
| Previous pacemaker | 0 (0) |
| Previous conduction disturbances | 0 (0) |
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Sutureless valve and size | Perceval S19-21 mm | Perceval S19-21 mm | Perceval S19-21 mm | Perceval M21-23 mm | Perceval XL 25-27 mm |
| Failure time from SAVR, y | 0.96 | 3.12 | 3.42 | 4.96 | 2.68 |
| Mechanism of dysfunction | Stent creep | Stent creep | Cusp calcification/degeneration | Cusp calcification/degeneration | Aortic regurgitation |
| Preprocedure measurements | |||||
| Aortic gradients, mmHg | 98/69 | 75/56 | 78/49 | 77/48 | 21/11 |
| Aortic regurgitation grade (localization) | Mild (central) | Moderate(intraprosthetic) | Moderate(intraprosthetic) | Moderate(intraprosthetic) | Severe (intraprosthetic) |
| Annulus diameter (CT), mm | 20.7 | 19.3 | 18.5 | 20.8 | 25.4 |
| Annulus area (CT), mm3 | 317.9 | 303.7 | 251.9 | 356.4 | 512.4 |
| Annulus perimeter (CT), mm | 64.3 | 63.5 | 58.9 | 69.3 | 82.7 |
| Ostium heights (LCA/RCA), mm | 11.2/12.8 | 18.2/16.3 | 11.7/17.9 | 18.9/21.1 | 12/20 |
| VTC, mm | 5.3 | 4.6 | 4.5 | 5.5 | 4.5 |
| LVEF, % | 71 | 65 | 73 | 68 | 47 |
| Procedure characteristics | |||||
| TAVI valve and size | CoreValve Evolut PRO 23 mm | Symetis ACURATE neo 23 mm | Edwards SAPIEN 3 23 mm | Edwards SAPIEN 3 23 mm | Edwards SAPIEN 326 mm |
| ViV approach | Femoral | Femoral | Femoral | Femoral | Femoral |
| Predilation | 20mm, 16 atm | 18mm, 18 atm | No | No | No |
| Postdilation | 22mm, 14 atm | 18mm, 17 atm | No | No | No |
| Final aortic gradients, mmHg | 40/27 | 33/19 | 25/16 | 19/10 | 18/9 |
| Postprodedure leak | None | None | None | None | None |
| Follow-up of procedural complications | |||||
| Conduction disturbances | Intraprocedural AVB | Intraprocedural AVB | NO-LBBB | NO-LBBB | None |
| Pacemaker | Yes | Yes | No | No | No |
| Other complications | None | None | None | None | None |
| Follow-up | |||||
| Aortic gradients at 6 month-FU, mmHg | 26/15 | 30/17 | 20/10 | 15/7 | 16/7 |
AVB, atrioventricular block; BMI, body-mass index; CT, computed tomography; FU, follow-up; LCA, left coronary artery; LVEF, left ventricular ejection fraction; NO-LBBB, new-onset left bundle branch block; NYHA, class of the New York Heart Association; RCA, right coronary artery; SAVR, surgical aortic valve replacement; STS score, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation; ViV, valve-in-valve; VTC, virtual transcatheter heart valves to coronary distance.
The data are presented as absolute numbers, No. (%), mean±standard deviation, or max/mean.
A: computed tomography measurements (diameters, perimeter derived diameter, area and perimeter specified below each case) of annulus dimensions of failing sutureless aortic valves. B: final angiographic result of valve-in-valve procedures with different types of transcatheter heart valves in failing sutureless aortic valves.
Sutureless valves with stent invagination were predilated and postdilated using an Atlas Gold (CR Bard, Murray Hill, New Jersey) balloon catheter in order to make the implantation more predictable. Self-expandable valves (CoreValve Evolut PRO in 1 patient and Symetis ACURATE neo in 1 patient) were used due to their repositionable features and supra-annular design. An Edwards SAPIEN 3 was implanted in the other 3 patients without sutureless valve stent underexpansion with no need for pre- or postdilatation. All procedures were safely performed with good results (mean gradients <20mmHg with no residual leaks).
Although THV durability appears favorable in the setting of native aortic valve stenosis, there are concerns about its durability in underexpanded ViV implants and the occurrence of specific procedural complications. Certain surgical valves have been identified as having a higher risk of coronary obstruction (the main mechanism being occlusion of the ostia by a dislodged leaflet after deployment of the THV). Thus, detailed preprocedural computed tomography study is crucial to identify high-risk features (such as low coronary heights, shallow sinuses of Valsalva, and short virtual THV to coronary distance).4 Although very few reports are available, we extend this experience with 5 patients, none of whom developed coronary obstruction. Although the risk of coronary occlusion seemed low (high coronary heights, wide sinuses, and virtual THV to coronary distance> 4mm), this type of valve may have a lower risk of coronary occlusion than other prostheses due to its implantation technique and valve design (the metallic stent has sinusoidal struts that fit Valsalva sinuses). Regarding conduction disturbances, a recent propensity-matched analysis reported lower rates of pacemaker implantation with ViV vs redo surgery for the management of failed prostheses.5 Data on sutureless valves are lacking, but among our patients, 2 patients with Percevals with stent deformation treated with self-expandable valves presented with atrioventricular block requiring pacemaker implantation, 2 patients with Percevals S and M treated with Edwards SAPIEN 3 presented with new onset left bundle branch block that was treated conservatively, and 1 patient with Perceval XL did not have any conduction abnormalities.
One of the advantages of sutureless valves is the absence of a sewing ring, which provides larger EOAs and reduces the risk of the development of patient-prosthesis mismatch compared with other bioprostheses. As described in table 1, acceptable gradients where achieved in our patients after the procedure and at mid-term follow-up. Paravalvular leaks were not a major concern in any of the patients in our series.
To conclude: a) ViV transcatheter aortic valve implantation in sutureless valves was feasible and safe; b) challenging cases such as small degenerated valves were successfully treated with self-expandable valves and acceptable gradients, and c) the rate of procedural complications was low and good in-hospital and mid-term outcomes were acheived with different types of transcatheter aortic valves.
Currently, procedural or mid-term results for ViV procedures in sutureless aortic valves are lacking. However, because sutureless valves are becoming more widely used and ViV is increasing, thorough knowledge will be essential and the features described above provide insights into the safety and feasibility of these procedures.