Transcatheter closure has become a common and preferred minimally invasive procedure for patent foramen ovale (PFO) and secundum atrial septal defect (ASD), especially in the treatment of secundum ASD, in which there has been a shift away from invasive surgery.1 In patients aged 18 to 60 years with prior PFO-associated stroke, transcatheter PFO closure is favored over antiplatelet therapy alone.2 However, nickel allergy, affecting up to 20% of the general population, poses a challenge in patients requiring transcatheter closure, as most approved occluder devices for PFO/ASD contain nitinol, an alloy of nickel and titanium.3
Despite being relatively rare (1 per 17 000) in PFO/ASD patients, device-related allergic events are considered serious, potentially requiring surgical removal.4 The Gore PFO/ASD occluder (Gore Medical, United States) demonstrates in vitro nickel elution levels similar to placebo, with significantly lower nickel exposure than other approved devices, such as the Amplatzer device (Abbott Laboratories, United States).5 As a result, the Gore device may be a suitable alternative in patients with established cutaneous nickel allergy. Limited real-world evidence exists on the safety of Gore devices in ASD/PFO patients with proven nickel allergy due to the rarity of this complication. This study explores the experience of a high-volume center with ASD/PFO closure using Gore devices in adult patients with cutaneous nickel allergy, and describes both short- and long-term outcomes.
We reviewed the electronic medical records of our hospital to identify patients who underwent transcatheter closure of ASD/PFO between 2012 and 2023, in which the Gore device was used, and conducted a retrospective chart review to identify if the device choice was due to cutaneous nickel allergy. Per current practice protocol, each patient is asked if they have a known nickel allergy, after which those who respond positively are referred to dermatology for confirmatory nickel hypersensitivity testing through a skin patch test. This test involved placing nickel sulfate hexahydrate 2.5% in petrolatum on ScanporR tape.
The follow-up protocol at the center included an in-person clinical visit at 3 to 6 months with a transthoracic echocardiogram after the index procedure and at 1 year, after which most patients returned to community care. The last follow-up data available were obtained by reviewing medical records. The study protocol was approved by the institutional review board.
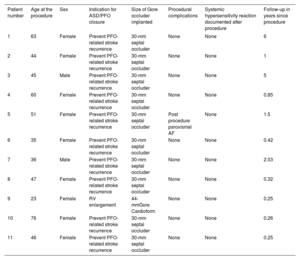
Among 2054 patients, 50 with possible nickel allergy requiring transcatheter closure for PFO or ASD underwent formal cutaneous nickel allergy testing. Overall, 11 of them had proven nickel allergy and were treated with a Gore device. The indication for transcatheter correction was secundum ASD with right ventricular enlargement in 1 patient and prevention of PFO-related stroke recurrence in the remaining patients. The baseline characteristics are shown in table 1. Most patients were female (82%), and the mean age was 52 years (standard deviation±15). The 30-mm Gore septal PFO occluder was used for PFO closure, and the 44-mm Gore Cardioform was used for ASD closure. Regarding periprocedural complications, only 1 patient who underwent PFO closure had a transient episode of atrial fibrillation.
Procedural characteristics and follow-up
| Patient number | Age at the procedure | Sex | Indication for ASD/PFO closure | Size of Gore occluder implanted | Procedural complications | Systemic hypersensitivity reaction documented after procedure | Follow-up in years since procedure |
|---|---|---|---|---|---|---|---|
| 1 | 63 | Female | Prevent PFO-related stroke recurrence | 30-mm septal occluder | None | None | 6 |
| 2 | 44 | Female | Prevent PFO-related stroke recurrence | 30-mm septal occluder | None | None | 1 |
| 3 | 45 | Male | Prevent PFO-related stroke recurrence | 30-mm septal occluder | None | None | 5 |
| 4 | 60 | Female | Prevent PFO-related stroke recurrence | 30-mm septal occluder | None | None | 0.85 |
| 5 | 51 | Female | Prevent PFO-related stroke recurrence | 30-mm septal occluder | Post procedure paroxismal AF | None | 1.5 |
| 6 | 35 | Female | Prevent PFO-related stroke recurrence | 30-mm septal occluder | None | None | 0.42 |
| 7 | 36 | Male | Prevent PFO-related stroke recurrence | 30-mm septal occluder | None | None | 2.03 |
| 8 | 47 | Female | Prevent PFO-related stroke recurrence | 30-mm septal occluder | None | None | 0.32 |
| 9 | 23 | Female | RV enlargement | 44-mmGore Cardioform | None | None | 0.25 |
| 10 | 76 | Female | Prevent PFO-related stroke recurrence | 30-mm septal occluder | None | None | 0.26 |
| 11 | 46 | Female | Prevent PFO-related stroke recurrence | 30-mm septal occluder | None | None | 0.25 |
AF, atrial fibrillation; ASD, atrial septal defect; PFO, patent foramen ovale; RV, right ventricle.
All patients underwent a transthoracic echocardiogram within 3 to 6 months after the procedure, and a bubble test was performed for those who underwent transcatheter PFO closure. The bubble test was negative for all 10 postclosure patients, and color Doppler ruled out a shunt across the ASD device in the remaining patients.
Median follow-up was 1.25 [interquartile range, 2.88] years. None of the patients experienced a systemic hypersensitivity reaction after transcatheter closure during follow-up. A total of 2 patients had venous thromboembolism after PFO closure during the follow-up period, and 1 had surgical aortic valve replacement.
This case series highlights the safety and effectiveness of a 2-step approach in patients with reported cutaneous nickel allergy. The first step involves nickel patch testing to confirm the contact sensitization/allergy before proceeding with transcatheter ASD/PFO closure. The second step involves the implantation of Gore devices. Of note, there were no reports of systemic nickel-related hypersensitivity reactions during follow-up in our case series, underscoring the safety of Gore devices in this context. There is ongoing debate regarding the development of nickel hypersensitivity syndrome after PFO/ASD occlusion with current devices in patients with known cutaneous nickel allergy. Both Amplatzer and Gore devices, currently the most extensively used devices for ASD/PFO closure worldwide, release nickel into the bloodstream during the first postprocedural months. At 90 days, nickel elution of the Amplatzer device has proven to be higher than that with the Gore.5 Notably, a cause-effect relationship has not been documented in the literature. Indeed, there is doubt about the true incidence or even the existence of nickel hypersensitivity. However, new-onset atrial fibrillation, especially in the first 1 to 3 months, has been proposed in a wide range of manifestations of nickel hypersensitivity. Theoretically, nickel hypersensitivity could create systemic inflammation that could provoke arrhythmogenesis until complete endothelization of the device.6 Concerns about the safety of using medical devices containing nickel have led the US Food and Drug Administration to warn about possible allergic reactions in patients with nickel allergy. However, the European position paper on managing PFO and the Society for Cardiovascular Angiography and Interventions (SCAI) guidelines for managing PFO do not address nickel allergy and its potential complications in patients requiring device closure.
As most cases describing nickel hypersensitivity due to ASD/PFO occlusion have been documented mainly with nickel patch testing, it seems prudent to perform this test preimplantation to confirm nickel allergy in patients with a compatible history and then to choose devices with less nickel and release like Gore. Indeed, a recent meta-analysis suggests that nickel allergy confirmed on a patch test may be associated with an increased risk of adverse outcomes following implantation of a nickel-containing device, including 2 studies involving PFO/ASD closure with Amplatzer devices.3
The INSPIRE trial (NCT04713683) may help answer this clinical question. The trial is enrolling patients with PFO-related stroke who undergo skin patch tests for nickel and other allergens 14 days before the procedure. They are then randomized to receive an Amplatzer PFO Occluder or a Gore Septal Occluder. Outcomes are then measured 90 days postprocedure, including changes in the nickel patch test results, residual leakage, and patient-reported symptoms.
It is important to recognize the limitations of our study, including its small sample size and single-center design, and that more than half of the included patients had a relatively short follow-up, specifically less than 1 year. However, considering the limited evidence, our study provides data on the safety and efficacy of a 2-step strategy for individuals with documented cutaneous nickel allergy who require closure of PFO/ASD. Initial nickel patch testing was conducted to verify cutaneous allergy, followed by the subsequent closure with a Gore device.
FUNDINGE. Horlick is supported by the Peter Munk Chair in Structural Heart Disease Intervention.
ETHICAL CONSIDERATIONSThe study protocol was approved by the institutional review board.
Informed consent was obtained from all patients and archived for the publication of their data.
In this work the possible variables of sex and gender were taken into account in accordance with the SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence was used in the preparation of this work.
AUTHORS’ CONTRIBUTIONSE. Flores-Umanzor and E. Horlick conceived and designed the analysis. E. Flores-Umanzor, and L. Abrahamyan performed the analysis. L. Abrahamyan, E. Horlick, M. Osten, L. Benson and J. DeKoven reviewed and edited the manuscript.
CONFLICTS OF INTERESTNone.