Stroke prevention following successful catheter ablation of atrial fibrillation remains a controversial topic. Oral anticoagulation is associated with a significant reduction in stroke risk in the general atrial fibrillation population but may be associated with an increased risk of major bleeding, and the benefit: risk ratio must be considered. Improvement in successful catheter ablation and widespread use of cardiac monitoring devices may allow for novel anticoagulation strategies in a subset of patients with atrial fibrillation, which may optimize stroke prevention while minimizing bleeding risk. In this review, we discuss stroke risk in atrial fibrillation and the effects of successful catheter ablation on thromboembolic risk. We also explore novel strategies for stroke prevention following successful catheter ablation.
Keywords
Atrial fibrillation (AF) is characterized by an irregular heart rhythm that is associated with an excess risk of stroke, major cardiovascular events, heart failure, and mortality.1 The 3 pillars of AF management are: a) stroke prevention; b) better symptom control with patient-centered symptom-directed rate or rhythm control; and c) cardiovascular risk factor and lifestyle management.2 This holistic approach, known as the ‘atrial fibrillation better care’ (ABC) pathway, is related to better clinical outcomes in various retrospective and prospective trial cohorts.3,4
Although oral anticoagulation (OAC) is the cornerstone of stroke prevention,5,6 rhythm control with catheter ablation has also been associated with reduced stroke risk.7 Current guidelines recommend OAC prescription based on the CHA2DS2-VASc score,8–10 but it is unclear how well this approach fits with the modern cohort of AF patients following AF ablation, especially those whose AF burden is much reduced.
It is therefore important to consider whether continuous long-term OAC—which, although generally safe, in some cases may lead to life-threatening bleeding11,12—is warranted following successful ablation. In this narrative review, we will discuss 3 novel approaches to stroke prevention.
ATRIAL FIBRILLATION, STROKE RISK, AND THE EFFECT OF CATHETER ABLATIONBefore discussing specific approaches to stroke prevention, it is important to set the scene. We will first review the association between AF and stroke, the effects of rhythm control, and current guideline recommendations.
ATRIAL FIBRILLATION AND PROTHROMBOTIC RISKAF confers an increased thrombotic risk by fulfilling all 3 aspects of Virchow's triad of thrombosis. Stasis of blood flow occurs because AF prevents atrial contraction. This particularly affects the left atrial appendage, where it is estimated that 90% of AF-related thrombi develop.13 Additionally, biomarkers reflecting both prothrombosis and endothelial injury are elevated in AF.13
While cardiovascular comorbidities are associated with an elevated prothrombotic risk, the presence of AF itself independently increases this risk.13 Patients with AF and no other thromboembolic risk factors have been found to have higher levels of prothrombotic biomarkers compared with matched controls.14–16 Patients with paroxysmal AF may have activation of the coagulation cascade and evidence of endothelial injury within a few hours of arrhythmia onset; these changes were shown to be dependent on the duration of paroxysmal AF but not age, sex, body mass index, or CHA2DS2-VASc score.17
Furthermore, restoration of sinus rhythm with cardioversion may reduce platelet reactivity within 4 weeks,18 and sinus rhythm maintenance with catheter ablation may improve global thrombotic status with enhanced fibrinolysis; this change was not observed among patients who had AF recurrence.19
ARRHYTHMIA BURDEN, STROKE, AND CATHETER ABLATIONOver the past decade, there has been increasing use of cardiac monitoring and implantable cardiac devices, thus introducing the concept of ‘AF burden’, ie, the proportion of time an individual spends in AF. AF burden is an important marker of stroke and mortality risk, despite frequently being asymptomatic.20–22 A pooled analysis of 5 prospective studies found that device-detected AF burden was linked to increased ischemic stroke risk over 24 months of follow-up.23 In the KP-RHYTHM study of 1965 nonanticoagulated patients with paroxysmal AF, patients with the highest tertile of AF burden had a 3-fold higher adjusted rate of thromboembolism compared with the combined lower 2 tertiles.24 More recently, 2 large randomized controlled trials on the effects of anticoagulation for device-detected AF lasting for 6minutes or more found differing results.25,26 Nonetheless, a meta-analysis of study-level data from these trials demonstrated that OAC with edoxaban or apixaban reduces the risk of stroke and increases the risk of bleeding in patients with device-detected AF.27
Catheter ablation has been proven to reduce the burden of AF compared with medical therapy in patients with both paroxysmal28,29 and persistent AF,30,31 and is superior to antiarrhythmic drug therapy in reducing AF recurrence.32 Moreover, ablation also delays the progression of AF from paroxysmal to persistent, which would otherwise elevate the risk of stroke.33,34 For example, a randomized controlled study of 225 patients found that 2.4% of ablated patients progressed from paroxysmal to persistent AF compared with 17.5% of those treated with antiarrhythmic drugs (P=.0009).35 Similarly, in the EARLY-AF trial, 1.9% of 303 patients initially treated with cryoballoon ablation had an episode of persistent AF compared with 7.4% on medical therapy (hazard ratio [HR], 0.25; 95% confidence interval [95%CI], 0.09-0.70).36 Similar findings were shown in those who required repeat ablation.37
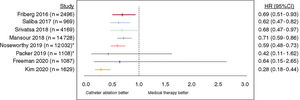
STROKE RISK FOLLOWING CATHETER ABLATIONRecent studies suggest that catheter ablation may be effective in reducing stroke risk (figure 1). In a propensity score matched study of 8145 patients (1:2:2 for AF ablation cohort, medical therapy cohort, and non-AF participants), the incidence rate (per 100 person-years) of ischemic stroke was significantly lower among patients treated with catheter ablation compared with medical therapy (0.3% vs 1.09%; P<.001) and was similar to patients without AF (0.34%; P=.673 vs ablation).38 Furthermore, patients who remained in sinus rhythm following catheter ablation had a lower stroke risk compared with those with AF recurrences, suggesting that the quality of AF control is important. A large retrospective database study of 183 760 patients with AF found catheter ablation was linked to a significant reduction in the risk of ischemic stroke (HR, 0.59; 95%CI, 0.48-0.73; P<.001).39 Similar findings have been described in other observational studies with either catheter ablation and/or antiarrhythmic medication use,7,40,41 suggesting that the maintenance of sinus rhythm plays a role in curtailing stroke risk, particularly when rhythm control is initiated early (ie,<3 months).
Studies demonstrating the effect of catheter ablation vs medical therapy on risk of stroke. 95%CI, 95% confidence interval; HR, hazard ratio; *unadjusted hazard ratio. Reproduced with permission from Ding et al.7
To date, there are no sufficiently powered randomized controlled trials investigating the effects of catheter ablation on AF stroke risk. The on-going OCEAN trial, estimated to be completed in late 2025, will assess rivaroxaban vs aspirin in patients without AF recurrence 12 months postablation and may help to inform strategies going forward.42
CURRENT GUIDELINES FOR ANTICOAGULATIONContemporary guidelines do not distinguish between patients with unablated AF and those in sinus rhythm postablation. While these patients almost certainly differ in terms of stroke risk, it remains unclear where the threshold for OAC therapy should lie.
Equally, most studies on catheter ablation report 1 to 2-year outcomes, with a scarcity of evidence for longer-term maintenance of sinus rhythm. Ablation success rates are modest in the setting of persistent AF,43 and hence there is concern that undetected AF recurrences may covertly increase stroke risk.44
Therefore, the guidelines are reasonably cautious and do not recommend cessation of long-term OAC despite successful catheter ablation among higher-risk patients.45 This decision is, however, not without danger, as OAC therapy confers a risk of major, potentially life-threatening bleeding.46–50
NOVEL STRATEGIES FOR STROKE PREVENTIONDiscontinuation of anticoagulation following successful catheter ablationThe first novel strategy may be to discontinue OAC entirely following successful catheter ablation. This minimizes the risk of bleeding and may improve quality of life by reducing anxiety, pill burden, and concerns around invasive procedures. Many electrophysiologists are already implementing this approach.51–53 For example, a retrospective study of 21 595 patients in the ORBIT-AF registry found that around 1 in 4 patients with CHA2DS2-VASc score ≥ 2 discontinued OAC, most frequently 2 months postablation.52 How does current evidence support this strategy?
Two meta-analyses found no difference in thromboembolism between patients who discontinued vs those who continued OAC following successful ablation,54,55 even with stratification by CHA2DS2-VASc score.55 Yang et al.56 concluded that it may be safe to discontinue OAC in postablation patients under close monitoring, in the absence of AF recurrence, history of stroke or systemic embolism, and diabetes mellitus. These studies should be interpreted with caution. In all 3 cases, point estimates suggested a trend toward reduced stroke risk with OAC discontinuation. This is likely explained by bias in the underlying populations: in both the meta-analyses and the study by Yang et al.,56 risk factors were higher in the OAC-continuation groups, likely reflecting physician preference to continue OAC in those at highest thromboembolic risk. Indeed, 2 other meta-analyses found that continuation of long-term OAC significantly decreased thromboembolic risk in patients with a CHA2DS2-VASc score of ≥ 2.57,58 All of these studies were consistent in terms of bleeding risk, which was significantly reduced with discontinuation of OAC, again highlighting the potential safety implications.
An important issue to consider is the management of recurrent arrhythmia postablation. This is not relevant in the immediate postablation period during which anticoagulation is mandated. However, patients who have discontinued anticoagulation and who have highly symptomatic arrhythmia recurrences and may require cardioversion may need a transoesophageal echocardiogram, or cross-sectional imaging with associated X-ray exposure, to exclude appendage thrombus. Luckily, this is a rare situation and not needed in patients presenting within 48hours of arrhythmia recurrence.
Given conflicting meta-analyses and the absence of high quality randomized controlled trials, the evidence base for widespread nontargeted OAC discontinuation following catheter ablation appears dubious. However, complete discontinuation is not the only way to manage OAC following rhythm control.
INTERMITTENT (PILL-IN-THE-POCKET) ANTICOAGULATIONAn intermediate strategy between long-term continuation and complete discontinuation is intermittent OAC. As described earlier, prothrombotic biomarkers are elevated early following the onset of AF. If AF recurrence postablation can be reliably monitored, this creates the potential for a ‘pill-in-the-pocket’ OAC approach. Such an approach is often used with antiarrhythmics, such as flecainide, whereby the patient simply takes a dose when their symptoms occur. This is not a routine strategy for anticoagulation in current practice, although it has some advocates.59
Such an approach is reliant upon a number of factors, in particular: a) how can we reliably monitor AF recurrence in the outpatient setting?; b) what is the evidence for a temporal association between AF onset and stroke?; and c) what is the optimum threshold for starting and stopping OAC? We address these aspects below.
MONITORING RECURRENCE: SMART WEARABLESWith the introduction and widespread uptake of smart wearables, there are better monitoring systems in place to allow for the detection of asymptomatic recurrence of AF postablation.60 Many of these technologies have integrated systems to determine AF burden. In the Apple Heart Study, the application of a smartwatch-based monitoring approach was successful in detecting AF among an unselected cohort of patients with no prior diagnosis of the condition.61 Large-scale studies using other technologies, such as the Huawei wristband or wristwatch62 or Fitbit devices63 for the detection of AF, have also been reported. These devices may permit early detection of AF recurrence postablation and may therefore inform the reintroduction of OAC therapy.
However, these devices have several limitations.59,64 First, they do not provide continuous passive cardiac monitoring. Instead, intermittent checks for pulse irregularity are made, with user notifications when certain thresholds are met. This may miss shorter AF episodes, although their relevance is debated. Second, as the devices are worn during everyday life, they are often affected by movement artifact, ie, many will suspend analysis when excessive artifact is detected. Finally, these devices require long-term patient adherence to reliably detect AF, which may not be suitable for all.
THE TEMPORAL ASSOCIATION BETWEEN ATRIAL FIBRILLATION AND STROKEAlthough prothrombotic biomarkers rise early after onset of AF, as described earlier, AF itself does not always precede stroke in a logical, temporal fashion. In a study of 51 patients with implantable devices who experienced a stroke or systemic embolism, 92% had no AF detected within the 30 days preceding their event.65 These strokes were not subclassified, and therefore the true incidence of cardioembolic stroke is uncertain. Two larger studies using continuous cardiac monitoring found that, while an episode of AF ≥ 5.5hours conferred a transient increase in stroke risk, most patients (approximately 3 in 4) had no evidence of AF ≥ 6minutes in the 120 days before their ischemic stroke.66,67
Additionally, a randomized controlled trial investigating a strategy of early initiation and interruption of OAC therapy based on remotely detected AF was terminated early as it did not improve outcomes compared with conventional treatment, partly due to the temporal dissociation between AF and stroke events.68 However, that study relied heavily on the use of vitamin K anticoagulants, which have a longer onset and offset than modern nonvitamin K antagonist oral anticoagulants (NOACs). Pilot studies have demonstrated the feasibility of implantable monitor-guided NOAC administration,69,70 but the safety and effectiveness of this approach has not yet been proven.
The randomized, noninferiority “REACT-AF” trial (NCT05836987) will begin recruitment of 5350 United States patients this year.59 The trial will compare smartwatch-guided NOAC vs long-term NOAC and will hopefully provide more data to support this concept.
INTERMITTENT ANTICOAGULATION THRESHOLDSFor a pill-in-the-pocket strategy to be effective, there must be a defined threshold for initiating temporary OAC. A number of studies have used a 5.5 hour threshold, based upon the TRENDS study.71 That study reported an approximate doubling of stroke risk in patients with ≥ 5.5hours AF burden in a given 30-day period, although it should be noted that this did not meet traditional statistical significance (HR, 2.20; 95%CI, 0.96-5.05; P=.06). Additionally, 5.5-hours was the median burden of patients with nonzero arrhythmia burden and was chosen due to the lack of prior evidence upon which to dichotomize the cohorts into low- and high-burden. In addition, AF burden varies over time, and may be related to progression to overt AF.72
If a pill-in-the-pocket approach were to be successful in clinical practice, a lower threshold may be required. This is partly to maximize the benefit in stroke prevention, accepting that this is a false dichotomy of a continuous scale, but mainly to minimize the intensity of monitoring required for detection. The higher the threshold, the more burdensome the monitoring required, which may adversely affect adherence.
Additionally, a threshold for discontinuing temporary OAC would be required. Studies have shown that, in patients with AF prior to ischemic stroke, thromboembolic risk is significantly elevated in the first 5 to 10 days.66 It is possible, then, that 10 days of OAC following an episode of AF lasting, perhaps, 2hours may be reasonable. A high quality prospective randomized trial would be required to prove the benefit of such an approach. At present, there are many unanswered questions that preclude the recommendation of intermittent anticoagulation in patients with AF.
COMBINING CATHETER ABLATION WITH LEFT ATRIAL APPENDAGE OCCLUSIONA third novel strategy, which may obviate the need to decide between long-term and intermittent OAC in selected patients, is to manage stroke risk during the ablation procedure using left atrial appendage occlusion (LAAO). This is based on the logic that most cardioembolic thrombi seen in AF develop in the left atrial appendage; therefore, isolating this structure should prevent thrombus migration to the rest of the body.
There is increasing interest among the electrophysiology community in performing AF ablation and LAAO as a combined procedure. Prior concerns with this approach included prolonged left atrial dwell time associated with older ablation technologies and increased early thrombotic risk due to both endothelial injury from ablation and device exposure to the blood pool. With the advent of Pulsed Field Ablation, which is both faster and safer than radiofrequency or cryoballoon techniques, the combined approach may be more feasible than ever.73 The combined procedure also minimizes the risk incurred by femoral access and transseptal puncture, which would need to be performed twice if the procedures were undertaken separately. Similarly, costs may be reduced by minimizing the total procedural time compared with 2 separate procedures.
A further advantage to such a ‘one-stop-shop’ approach is that it neatly addresses the above concerns around the temporal dissociation between AF and stroke. Regardless of temporality, the site of thrombus formation is still likely to be the LAA; therefore, by occluding the LAA and restoring sinus rhythm, it is possible that stroke risk can be minimized in a single procedure. This may also allow long-term discontinuation of anticoagulation,74 minimizing bleeding risk. A further benefit is that intermittent monitoring with smart wearables would be rendered unnecessary, reducing patient burden and improving quality of life.
Such an approach would require a large randomized controlled trial to prove benefit and cost-effectiveness before it could be recommended. This is because LAAO carries additional risk compared with ablation alone, such as device embolization or device-related thrombosis. Some risk is, however, offset by combining the 2 procedures, particularly the risk of femoral vascular injury and transseptal complications, as both are required for ablation even in the absence of LAAO.
LIMITATIONS AND INDIVIDUALIZED CAREJust as not all patients are suited to long-term OAC, it stands to reason that not every patient will be suitable for the novel strategies discussed above. Some patients may find long-term smart wearable monitoring burdensome or may be unable to use the requisite technologies. Some may prefer to continue OAC due to perceived stroke risk, while others may prefer long-term discontinuation due to perceived bleeding risk. Some may have left atrial appendage anatomy that is unsuitable for LAAO.
Additionally, AF is a marker of underlying atrial cardiomyopathy which, in itself, confers an increased stroke risk, even in the absence of AF.75–77 This is supported by evidence that a raised CHA2DS2-VASc score may predict ischemic stroke in individuals without diagnosed AF.78 For this reason, some physicians prefer to continue with OAC when rhythm control is successful in current clinical practice. Indeed, AF may be more of a risk factor for stroke, rather than the primary cause. While this factor should not preclude large-scale studies assessing novel OAC strategies, it does provide a plausible reason why such approaches may not work and may explain the temporal dissociation described earlier.
There may, therefore, be a threshold beyond which long-term OAC should be recommended in preference to novel strategies, even if these were proven in trials. The same may apply to patients with a high AF burden following unsuccessful ablation. These limitations may be less applicable to the combined ablation+LAAO approach, but the evidence base for this does not yet exist. There may also be a role for cardiac imaging in identifying patients at highest risk.
Individualized, shared decision-making remains key. If novel approaches to stroke prevention are to be beneficial, identifying appropriate patients and counseling them about the available options will be critical. Importantly, most of the evidence base relates to the type of AF known as “nonvalvular AF”, ie, AF which is not associated with moderate-to-severe mitral stenosis. Novel OAC strategies may not be applicable in patients with “valvular” AF. This review highlights the fact that there are numerous management strategies available that can be individualized to our specific patients.
Overall, there are logical reasons to expect stroke risk to remain elevated even when rhythm control of AF is successful. Equally, although rhythm control, particularly with catheter ablation, is highly successful in the short- to mid-term, very long-term follow-up is not possible in randomized controlled trials and recurrences decades after rhythm control must be considered. These factors, combined with the lack of high quality evidence, both for catheter ablation reducing stroke risk and for the novel approaches described above, make it difficult to recommend sweeping changes to current practice. However, another point is that the benefit of long-term OAC in the cohort of patients with successful catheter ablation for AF remains to be shown.
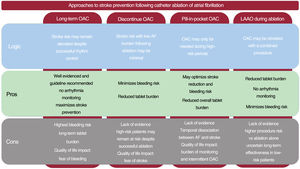
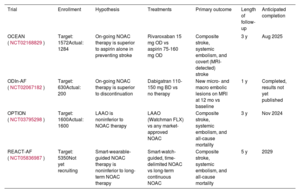
The stage is clearly set for high quality, randomized controlled studies. Four such on-going trials are summarized in table 1. A summary of the pros and cons of different stroke prevention strategies is presented in figure 2.
On-going studies of novel approaches to stroke prevention following catheter ablation of AF
| Trial | Enrollment | Hypothesis | Treatments | Primary outcome | Length of follow-up | Anticipated completion |
|---|---|---|---|---|---|---|
| OCEAN (NCT02168829) | Target: 1572Actual: 1284 | On-going NOAC therapy is superior to aspirin alone in preventing stroke | Rivaroxaban 15 mg OD vs aspirin 75-160 mg OD | Composite stroke, systemic embolism, and covert (MRI-detected) stroke | 3 y | Aug 2025 |
| ODIn-AF (NCT02067182) | Target: 630Actual: 200 | On-going NOAC therapy is superior to discontinuation | Dabigatran 110-150 mg BD vs no therapy | New micro- and macro embolic lesions on MRI at 12 mo vs baseline | 1 y | Completed, results not yet published |
| OPTION (NCT03795298) | Target: 1600Actual: 1600 | LAAO is noninferior to NOAC therapy | LAAO (Watchman FLX) vs any market-approved NOAC | Composite stroke, systemic embolism, and all-cause mortality | 3 y | Nov 2024 |
| REACT-AF (NCT05836987) | Target: 5350Not yet recruiting | Smart-wearable-guided NOAC therapy is noninferior to long-term NOAC therapy | Smart-watch-guided, time-delimited NOAC vs long-term continuous NOAC | Composite stroke, systemic embolism, and all-cause mortality | 5 y | 2029 |
AF, atrial fibrillation; BD, twice a day, MRI, magnetic resonance imaging; NOAC, nonvitamin K antagonist oral anticoagulant; OD, once a day.
Stroke prevention is a key aspect of successful AF management. Current guidelines are relatively one-dimensional, recommending long-term continuous OAC for patients with an elevated CHA2DS2-VASc score, regardless of residual AF burden following successful rhythm control. Novel management approaches may include: a) discontinuation of anticoagulation following successful catheter ablation; b) pill-in-the-pocket anticoagulation using smart wearables to detect AF; or c) a one-stop-shop approach combining catheter ablation with LAAO. High quality randomized controlled trials are needed to investigate these approaches.
FUNDINGNo funding was received for this work.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence has been used during the preparation of this work.
AUTHORS’ CONTRIBUTIONSW.Y. Ding and P. Calvert contributed equally to this work. W.Y. Ding and P. Calvert reviewed the literature, interpreted the data, and drafted and revised the manuscript. G.Y.H. Lip revised the manuscript. D. Gupta contributed to the conception of the study, and revised the manuscript. G.Y.H. Lip and D. Gupta are joint senior authors.
CONFLICTS OF INTERESTG.Y.H. Lip reports consultant and speaker fees for BMS/Pfizer, Boehringer Ingelheim, and Daiichi-Sankyo. No fees were directly received personally. D. Gupta reports speaker fees for Boehringer Ingelheim, Biosense Webster and Boston Scientific, has worked as a proctor for Abbott, and has received research grants from Medtronic, Biosense Webster, and Boston Scientific. The other authors report no conflicts of interest.