The emergence of the Perceval S sutureless biological aortic prosthesis (LivaNova, Italy) represents an advance in the development of replacement valves, especially for patients with moderate or high risk.1
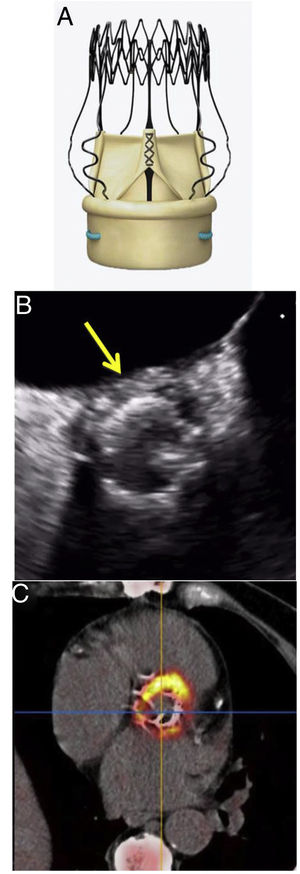
This new prosthesis is made of 3 bovine pericardial leaflets, mounted on a flexible, collapsible, external nitinol stent (figure 1). It allows a minimally-invasive approach and shortening of surgical times, as well as a reduction in postoperative morbidity and mortality. Its design improves the hemodynamic profile with better transvalvular gradients at follow-up and fewer cases of patient-prosthesis mismatch, making it the ideal replacement in patients with small aortic annulus or in complicated situations, such as infectious endocarditis.2 These advantages have led to its rapid and widespread uptake in multiple European cardiac surgery departments.
Infectious endocarditis is a disease with high morbidity and mortality, the most severe form being prosthetic valvular endocarditis (PVE). PVE occurs in approximately 1% to 6% of patients with a cardiac prosthesis and constitutes 10% to 30% of all cases of infectious endocarditis. Most patients require surgical treatment, but this is often a challenge, with significant perioperative morbidity and mortality.3
In the last 10 years, the Perceval S prosthesis has become the biological replacement valve of choice in our department, and is used in patients older than 70 years and with a moderate-high risk profile, with a mean follow-up close to 7 years and a low complication rate. We performed a retrospective observational study, with hospital ethics approval and patient consent, to analyze the prevalence and presenting characteristics of VPE in this type of prosthesis, with particular attention paid to diagnostic imaging. Since 2015, 670 Perceval S valves have been implanted and 14 cases of PVE have been diagnosed in them (2.1%). This figure represents 6.4% of all the PVE cases treated in our hospital during the same period (n=220).
Table 1 describes the clinical characteristics, microbiological profile, imaging findings, and outcomes of the 14 patients with PVE. It should be noted that 11 cases were early-onset PVE, occurring > 3 months after surgery, and the most common organism was Staphylococcus epidermidis (n=7).
Preoperative, clinical, and microbiological characteristics
AVR, aortic valve replacement; CKD, chronic kidney disease; CNS, coagulase-negative Staphylococcus; COPD, chronic obstructive pulmonary disease; IC, inconclusive; NA, not applicable (patient not suitable for surgery); PET/CT positron-emission tomography/computed tomography; SI, surgical intervention; TEE, transesophageal echocardiography; VGS, viridans group Streptococcus.
The data are expressed as No. (%) or mean ± standard deviation.
Transesophageal echocardiography was performed in all patients. In this investigation, periprosthetic leak was found in only 1 patient, vegetations were identified in 2 patients, and periannular thickening was observed in 7, but this was not diagnostic of an abscess (< 10mm).
Due to raised clinical suspicion, 10 patients underwent 18F-fluorodesoxyglucose positron-emission tomography/computed tomography (18F-FDG PET/CT). In patient 2, with no findings on transesophageal echocardiography or PET, the diagnosis was confirmed on post-mortem; in patient 4, 18F-FDG PET/CT was the first investigation because the patient had esophageal varices, and in patient 5 it was performed as part of the preoperative surgical planning. There was a diverse intensity of metabolic uptake among the patients, with anatomical depiction of periannular abscess in all of them (figure 1).
All 14 patients had an indication for surgery, but this was ruled out in 3 due to excessive risk. In 8 of the 11 patients who underwent surgery, a new Perceval S prosthesis was implanted after patching the aortic annulus with bovine pericardium. In all the patients who underwent surgery, the intraoperative findings correlated with those described on 18F-FDG PET/CT. Furthermore, the anatomical information provided with this technique facilitated planning of the most appropriate surgical strategy.
In-hospital mortality was 14% (n=2), and during follow-up (mean, 23 months) 5 patients died (41%), 2 of whom had previously been declined surgery.
The special design of the Perceval S prosthesis, anchoring the valve to the aortic annulus by means of the radial force of the nitinol stent alone, means that the echocardiographic presentation of endocarditis can differ from that of other valve replacements. The lack of periprosthetic leak could delay the diagnosis with transesophageal echocardiography, especially if the images of periannular involvement are not diagnostic of abscess.
Although transesophageal echocardiography is an essential tool in the diagnosis of PVE, a false negative rate of up to 10% has been described in the early stages of the disease.4 With this type of prosthesis, it can be helpful to use other imaging techniques, such as 18F-FDG PET/CT, a very useful diagnostic tool when there is suspicion of PVE, even in the early stages.5,6 Electrocardiogram-synchronized CT has very high sensitivity for detecting periannular abscesses (> 95%), which is increased with the metabolic information from PET.6
In our experience, presentation of PVE on a Perceval S valve differs from that on other biological prostheses when it comes to the echocardiographic diagnosis. The use of other techniques such as 18F-FDG PET/CT can facilitate an early diagnosis and better evaluation of periannular anatomical involvement. Such an approach could expedite treatment decisions and minimize the risk of severe local complications that would increase the complexity of subsequent surgery.
FUNDINGNo funding was provided.
CONFLICTS OF INTERESTNone.