For patients with acute coronary syndrome (ACS) treated with percutaneous coronary intervention (PCI), it is unclear whether angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) are associated with reduced mortality, particularly with preserved left ventricular ejection fraction (LVEF). The goal of this study was to determine the association between ACEI/ARB and mortality in ACS patients undergoing PCI, with and without reduced LVEF.
MethodsData from the BleeMACS registry were used. The endpoint was 1-year all-cause mortality. The prognostic value of ACEI/ARB was tested after weighting by survival-time inverse probability and after adjustment by Cox regression, propensity score, and instrumental variable analysis.
ResultsAmong 15 401 ACS patients who underwent PCI, ACEI/ARB were prescribed in 75.2%. There were 569 deaths (3.7%) during the first year after hospital discharge. After multivariable adjustment, ACEI/ARB were associated with lower 1-year mortality, ≤ 40% (HR, 0.62; 95%CI, 0.43-0.90; P=.012). The relative risk reduction of ACEI/ARB in mortality was 46.1% in patients with LVEF ≤ 40%, and 15.7% in patients with LVEF> 40% (P value for treatment-by-LVEF interaction=.008). For patients with LVEF> 40%, ACEI/ARB was associated with lower mortality only in ST-segment elevation myocardial infarction (HR, 0.44; 95%CI, 0.21-0.93; P=.031).
ConclusionThe benefit of ACEI/ARB in decreasing mortality after an ACS in patients undergoing PCI is concentrated in patients with LVEF ≤ 40%, and in those with LVEF> 40% and ST-segment elevation myocardial infarction. In non–ST-segment elevation-ACS patients with LVEF> 40%, further studies are needed to assess the prognostic impact of ACEI/ARB.
Keywords
Angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) are part of the 4 therapies that are considered evidence-based medications after acute coronary syndromes (ACS), along with dual antiplatelet therapy (DAPT), beta-blockers, and statins.1,2 However, the evidence on the benefit of ACEI/ARB comes from a clinical scenario that is very different from the current one, with a very low rate (< 20%) of percutaneous coronary intervention (PCI) and treatment with DAPT and statins.3–7 With the exception of patients with reduced left ventricular ejection fraction (LVEF) ≤ 40%,) where the evidence is large and consistent8–10—complemented by trials conducted in the field of heart failure (HF)11,12—, the evidence on the prognostic impact of ACEI/ARB in patients with LVEF> 40% treated with PCI, DAPT, and statins is scarce and also contradictory.13–16 Despite this obsolete and outdated evidence, clinical practice guidelines continue to favor the use of ACEI/ARB in all post-ACS patients, beyond patients with LVEF ≤ 40%.1,2
Taking into account this “time gap” between the clinical trials and the current guideline recommendations, the availability of exploratory data analyzing whether the results of pre-PCI trials are still valid in the current era of PCI should be of great clinical value. In this study, we aimed to analyze the impact of post-ACS ACEI/ARB in patients undergoing PCI and treated according to current recommendations, with high rates of DAPT, beta-blockers, and statins.
METHODSStudy design and populationThe analyses were based on data from the BleeMACS registry. BleeMACS is a retrospective, observational, multicenter cohort registry involving 15 401 consecutive patients. The inclusion and exclusion criteria, data collection, and variables have been described previously.17 Briefly, eligible patients were all consecutive adult patients (≥ 18 years) discharged with a definitive diagnosis of ACS, defined according to clinical guidelines1,2 as evidence of significant coronary artery disease on coronary angiography (stenosis ≥ 50% in left main coronary artery or ≥ 70% in the rest of the coronary arteries) and who underwent in-hospital PCI, with follow-up data for 1 year. Participants were recruited from 15 hospitals from North and South America (Canada and Brazil), Europe (Germany, Poland, the Netherlands, Spain, Italy, and Greece), and Asia (China and Japan). Enrollment was conducted from November 2003 through June 2014. More information about the BleeMACS design and study population is shown in the supplementary data (Information about BleeMACS registry). The study protocol conforms to the ethics guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval of the ethics committee of each center. The study was registered in ClincalTrial.gov (NCT02466854).
Follow-up and outcomesThe primary outcome was all-cause mortality, with comparison of patients treated with ACEI/ARB at discharge vs those not treated. The prescription of ACEI/ARB was based on the clinical judgment of the treating physician. All patients were systematically followed up for 1 year to assess vital status, ascertained by trained research coordinators at each participating site. Data on vital status were obtained from hospital and/or administrative data records, and/or by contacting the patients or their relatives by telephone, when necessary.
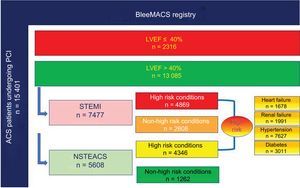
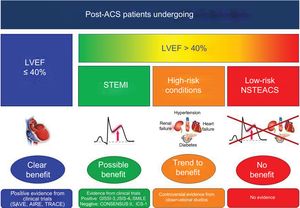
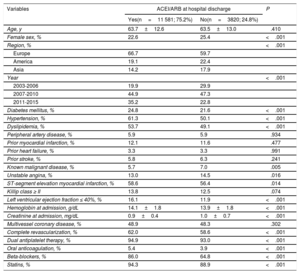
Statistical analysesThe statistical analysis was performed with SPSS version 24.0, R version 3.2.2, and Stata MP64 version 14. Baseline characteristics according to treatment with ACEI/ARB were described by using number and percentage for categorical data and standardized mean difference for continuous data, respectively. Differences in characteristics were assessed by using chi-square tests and 2-sample Student t tests. The association between ACEI/ARB exposure and 1-year death was studied in robust Cox proportional hazards models with adjustment for potential confounders at baseline. The multivariable model risk adjustment was performed with all variables associated with postdischarge mortality based on clinical plausibility or P value <.05 in the univariate Cox analyses (univariate cox analysis and Table 1). Because of important differences in key baseline characteristics depending on prescription of ACEI/ARB therapy (Table 1), we complemented this analysis using a propensity score (PS) analysis. PS were estimated using a nonparsimonious multivariable logistic regression model, with ACEI/ARB therapy as the dependent variables and those characteristics that differed between patients treated and not treated with ACEI/ARB (Table 1) as covariates. Subsequent PS matching was performed to assemble a cohort in which all the measured covariates would be well balanced across the comparator group (additional details are presented in propensity score analysis, Table 2 and Figures 2-4). In the PS-matched population (constituted by 2 groups of 3 765 patients with similar characteristics according to prescription of ACEI or not), the reduction in death rate was compared using a robust stratified Cox regression model. Survival-time inverse probability weighting propensity score analysis (IPW) was also used to evaluate the association between ACEI/ARB use and mortality. The effect of ACEI/ARB was graphically represented in Kaplan-Meier curves adjusted by IPW, to balance the covariate distribution between the treatment and control observations, and by those covariates associated with postdischarge mortality in the univariate Cox analyses, to further mitigate residual confounding in the survival modeling. We complemented the analysis with an augmented IPW (AIPW) to estimate the average treatment effects (ATEs), in a doubly robust method that combined both the properties of the regression-based estimator and the IPW estimator. The ATE coefficient assesses the absolute risk difference in 1-year death rate between patients treated and not treated with ACEI/ARB. Given that propensity scoring adjusts for measured confounding only, an instrumental variable analysis with annual rates of each hospital for the prescription of guideline-indicated treatments (DAPT, beta-blockers, statins, and ACEI/ARB) as the instrument was used to further assess potential selection bias (more information in instrumental variable analysis and ). The coefficient of instrumental variable analysis shows the relative risk reduction in death rate with ACEI/ARB. Analyses were undertaken for the overall ACS cohort and separately for cases with LVEF ≤ 40% and> 40% (the cutoff for LVEF of 40% was based on European guideline recommendations for acute coronary syndrome1,2). In the group of patients with LVEF> 40%, we separately analyzed patients with ST-segment elevation myocardial infarction (STEMI) and high-risk conditions (HF, renal failure, diabetes mellitus [DM], arterial hypertension) (Figure 1). These high-risk conditions were defined based on recommendations of European and American guidelines for treatment of ACS.1,2 An interaction test was carried out as part of the Cox regression models performed in the study to assess the treatment-by-subgroup interaction. A P-value <.05 was considered statistically significant.
Baseline characteristics according to prescription or not of ACEI/ARB at discharge
| Variables | ACEI/ARB at hospital discharge | P | |
|---|---|---|---|
| Yes(n=11 581; 75.2%) | No(n=3820; 24.8%) | ||
| Age, y | 63.7±12.6 | 63.5±13.0 | .410 |
| Female sex, % | 22.6 | 25.4 | <.001 |
| Region, % | <.001 | ||
| Europe | 66.7 | 59.7 | |
| America | 19.1 | 22.4 | |
| Asia | 14.2 | 17.9 | |
| Year | <.001 | ||
| 2003-2006 | 19.9 | 29.9 | |
| 2007-2010 | 44.9 | 47.3 | |
| 2011-2015 | 35.2 | 22.8 | |
| Diabetes mellitus, % | 24.8 | 21.6 | <.001 |
| Hypertension, % | 61.3 | 50.1 | <.001 |
| Dyslipidemia, % | 53.7 | 49.1 | <.001 |
| Peripheral artery disease, % | 5.9 | 5.9 | .934 |
| Prior myocardial infarction, % | 12.1 | 11.6 | .477 |
| Prior heart failure, % | 3.3 | 3.3 | .991 |
| Prior stroke, % | 5.8 | 6.3 | .241 |
| Known malignant disease, % | 5.7 | 7.0 | .005 |
| Unstable angina, % | 13.0 | 14.5 | .016 |
| ST-segment elevation myocardial infarction, % | 58.6 | 56.4 | .014 |
| Killip class ≥ II | 13.8 | 12.5 | .074 |
| Left ventricular ejection fraction ≤ 40%, % | 16.1 | 11.9 | <.001 |
| Hemoglobin at admission, g/dL | 14.1±1.8 | 13.9±1.8 | <.001 |
| Creatinine at admission, mg/dL | 0.9±0.4 | 1.0±0.7 | <.001 |
| Multivessel coronary disease, % | 48.9 | 48.3 | .302 |
| Complete revascularization, % | 62.0 | 58.6 | <.001 |
| Dual antiplatelet therapy, % | 94.9 | 93.0 | <.001 |
| Oral anticoagulation, % | 5.4 | 3.9 | <.001 |
| Beta-blockers, % | 86.0 | 64.8 | <.001 |
| Statins, % | 94.3 | 88.9 | <.001 |
ACEI/ARB, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers.
Analyses to assess the prognostic impact of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on 1-year mortality
| Population | Analysis | Complete cases | |
|---|---|---|---|
| Total population | |||
| Cox regression | HR | 95%CI | P |
| Univariate | 0.617 | 0.518-0.734 | < .001 |
| Multivariable* | 0.762 | 0.633-0.917 | .004 |
| Adjusted by IPW | 0.777 | 0.645-0.935 | .008 |
| After PS matching | 0.713 | 0.572-0.888 | .004 |
| AIPW | Coefficient | 95%CI | P |
| ATE (risk difference) | –0.008 | –0.015 to –0.001 | .034 |
| Instrumental variable | Coefficient | 95%CI | P |
| Relative risk reduction | –0.234 | –0.328 to –0.132 | .001 |
| Subgroups by LVEF | |||
|---|---|---|---|
| LVEF ≤ 40 | |||
| Cox regression | HR | 95%CI | P |
| Univariate | 0.397 | 0.284-0.555 | < .001 |
| Multivariable* | 0.620 | 0.428-0.899 | .012 |
| Adjusted by IPW | 0.523 | 0.357-0.765 | .001 |
| After PS matching | 0.417 | 0.262-0.665 | .001 |
| AIPW | Coefficient | 95%CI | P |
|---|---|---|---|
| ATE (risk difference) | –0.026 | -0.055 to 0.005 | .076 |
| Instrumental Variable | Coefficient | 95%CI | P |
|---|---|---|---|
| Relative risk reduction | –0.461 | -0.663 to - 0.259 | < .001 |
| Subgroups by LVEF | |||
|---|---|---|---|
| LVEF > 40 | |||
| Cox regression | HR | 95%CI | P |
| Univariate | 0.677 | 0.549-0.833 | < .001 |
| Multivariable* | 0.809 | 0.650-1.007 | .058 |
| Adjusted by IPW | 0.876 | 0.703-1.092 | .239 |
| After PS matching | 0.838 | 0.652-1.077 | .174 |
| AIPW | Coefficient | 95%CI | P |
|---|---|---|---|
| ATE (risk difference) | –0.005 | –0.012 to 0.001 | .121 |
| Instrumental Variable | Coefficient | 95%CI | P |
|---|---|---|---|
| Relative risk reduction | –0.157 | –0.264 to - 0.049 | .004 |
95%CI, 95% confidence interval; AIPW, augmented inverse probability weighting; ATE, average treatment effect; CI, confidence interval; HR, hazard ratio; IPW, inverse probability weighting; LVEF, left ventricular ejection fraction; PS, propensity score.
Adjustment for age, female sex, country, year, diabetes mellitus, hypertension, dyslipidemia, peripheral artery disease, prior myocardial infarction, prior heart failure, prior stroke, known malignant disease, unstable angina, ST-segment elevation myocardial infarction, Killip class ≥ II, left ventricular ejection fraction, hemoglobin at admission, creatinine at admission, multivessel coronary disease, complete revascularization, dual antiplatelet therapy, oral anticoagulation, beta-blockers, statins.
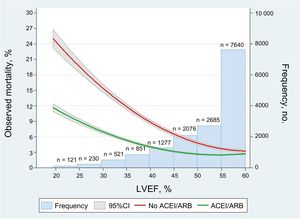
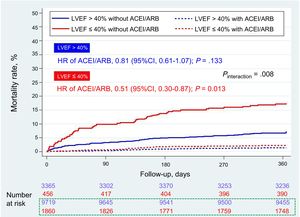
Adjusted survival Kaplan-Meier curves for the prescription of ACEI or ARB at discharge according to LVEF (left ventricular ejection fraction > 40% or ≤ 40%). 95%CI, 95% confidence interval; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; LVEF, left ventricular ejection fraction. Numbers at risk. In blue patients with left ventricular ejection fraction > 40%, and in red patients with LVEF ≤ 40%. In the box with green dashed line, patients treated with ACEI/ARB.
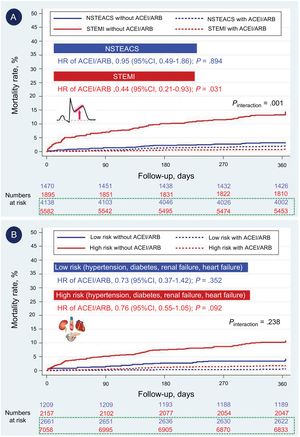
Adjusted survival Kaplan-Meier curves for the prescription of ACEI or ARB at discharge in patients with LVEF > 40%, according to: A) presence of STEMI or NSTEACS; B) high-risk conditions (heart failure, renal failure, diabetes mellitus, hypertension). 95%CI, 95% confidence interval; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; HR, hazard ratio; LVEF, left ventricular ejection fraction; NSTEACS, non–ST-segment elevation acute coronary syndrome; STEMI, ST-segment elevation myocardial infarction.
Study population according to LVEF (> 40% or ≤ 40%), type of ACS (STEMI or NSTEACS), and high-risk conditions (heart failure, renal failure, diabetes mellitus, hypertension). ACS, acute coronary syndrome; LVEF, left ventricular ejection fraction; NSTEACS, non–ST-segment elevation ACS; STEMI, ST-segment elevation myocardial infarction.
Missing data were handled by multiple imputations using the fully conditional specification method (an iterative Markov Chain Monte Carlo algorithm), generating 10 imputed datasets using all applicable adjustment variables and all outcome variables as predictors (). The effect of ACEI/ARB therapy on the mortality rate was computed separately in the 10 imputed data sets and then averaged over data sets using Rubin's combination rules (Imputed Datasets Analysis and ). Several sensitivity analyses were performed (additional details in Complete Case Analysis, ).
RESULTSPrescription of ACEI/ARBACEI/ARB were prescribed in 75.2% (n=11 581) of the 15 401 patients. There were significant differences in baseline characteristics between patients with and without ACEI/ARB (Table 1). In particular, patients who received ACEI/ARB tended to be at higher ischemic risk (including diabetes, hypertension, and dislipemia) compared with those not receiving ACEI/ARB. Patients treated with ACEI/ARB had worse renal function, but higher LVEF values, than patients not treated with ACEI/ARB, and the concomitant use of DAPT, beta-blockers, and statins was more frequent with ACEI/ARB prescription.
Benefit of ACEI/ARB in the total ACS populationOf the entire cohort (N=15 401), there were 569 deaths (3.7%) during the first year after hospital discharge. Unadjusted 1-year mortality was significantly lower in patients who received ACEI/ARB compared with those who did not (3.2% vs 5.1%, P <.001). After weighting and adjustment, using multivariable Cox analysis adjustment for those variables associated with mortality in the univariate analysis (), and for PS techniques (PS matching, IPW, and AIPW), treatment with ACEI/ARB continued to be associated with lower 1-year mortality (Table 2). Specifically, there was a significant absolute risk difference of 0.8% in 1-year postdischarge mortality between patients treated and not treated with ACEI/ARB, and a significant relative risk reduction of 1-year mortality of 23.4%, according to the AIPW regression adjustment and instrumental variable analysis, respectively (Table 2).
ACEI/ARB according to LVEFOf the 2316 patients (15.0%) with LVEF ≤ 40%, 1861 (80.4%) were treated with ACEI/ARB. Of the 13 085 patients (85.0%) with LVEF> 40%, 9720 (74.3%) were treated with ACEI/ARB. One-year mortality was 6.9% (n=161) and 3.1% (n=409) in patients with and without LVEF ≤ 40%, respectively. Therapy with ACEI/ARB was associated with a higher mortality reduction with lower LVEF (Figure 2). ACEI/ARB was strongly associated with lower mortality in patients with LVEF ≤ 40% after weighting and adjustment for the different methods (Table 2, Figure 3), with an absolute and relative mortality reduction of 2.6% (P value for ATE according to AIPW regression adjustment=.076) and 46.1% (P value for instrumental variable analysis <.001), respectively. For patients with LVEF> 40%, ACEI/ARB therapy was not associated with lower mortality in the various Cox analyses (Table 2, Figure 3), or with the AIPW regression adjustment (relative risk reduction of 0.5%, 95%CI,−1.2% to 0.1%). However, the coefficient of instrumental variable analysis showed a significant relative risk reduction of 15.7% (95%CI,−26.4% to−4.9%). The interaction P value for treatment-by-LVEF was significant (.008), indicating that the clinical benefit of ACEI/ARB was concentrated in patients with LVEF ≤ 40%. These results (from the imputed data cohort) were consistent with those observed in the case-complete cohort ().
Benefit of ACEI/ARB in STEMI and high-risk patients with LVEF> 40%
In the group of patients with LVEF> 40% (n=13,085), 7477 (57.1%) had STEMI (Figure 3). In these patients with STEMI, a specific robust Cox analysis, adjusted by IPW and by those variables associated with mortality in the univariate analysis, showed a lower 1-year mortality in patients treated with ACEI/ARB vs those not treated with ACEI/ARB (HR 0.44; 95%CI, 0.21-0.93; P=.031), which was not observed in patients with non–ST-segment elevation ACS (NSTEACS) (Figure 4A). The interaction P value for treatment-by-ACS type was significant (.001), indicating that the clinical benefit of ACEI/ARB in patients with LVEF> 40% was observed only in patients with STEMI (not in those with NSTEACS). Of the total number of patients with LVEF> 40%, 1678 (12.8%) had a history of HFdefined as prior admission for HF, and/or Killip class> I at ACS admission, and/or de novo HF during ACS hospitalization, 1991 (15.2%) had renal failure, defined as estimated glomerular filtrate rate by isotope dilution mass spectrometry (IDMS)—traceable Modification of the Diet in Renal Disease (MDRD) formula <60 mL/min/1.73 m2 at admission—, 3011 (20.0%) had DM, and 7627 (50.6%) had arterial hypertension. Of the subgroup of patients with LVEF> 40%, 9215 patients (61.1%) had at least 1 of these high-risk conditions. In this high-risk group, treatment with ACEI/ARB showed a trend to lower 1-year mortality (HR, 0.76; 95%CI, 0.55-1.05; P=.092) in comparison with the nonhigh-risk group (Figure 4B). However, the interaction P value for treatment-by-risk conditions was not significant (0.238), indicating that there are no differences in the clinical effect of ACEI/ARB in patients with LVEF> 40% by the presence or absence of high-risk conditions. The adjusted Kaplan-Meier curves for each high-risk condition are shown in , together with the case-complete analysis ().
DISCUSSIONIn this study, based on a large international registry of ACS patients undergoing PCI and treated according to current recommendations of ACS guidelines, we found a significant association of ACEI/ARB therapy with lower 1-year mortality. This association was centered on patients with LVEF ≤ 40% and with STEMI. ACEI/ARB also showed a trend to be associated with lower 1-year mortality in patients with HF, renal failure, DM, or hypertension. In NSTEACS patients without these high-risk conditions, we found no association of ACEI/ARB with lower death rate.
The benefit of ACEI/ARB in the setting of ACS has been probed in the thrombolysis era, with several favorable clinical trials3,5,7 and meta-analyses.18 However, there are 2 clinical trials, CONSENSUS II6 and CCS-14, with a thrombolysis rate of> 50%, that have not demonstrated a significant reduction in mortality at 6 months and 4 weeks, respectively, even in anterior infarction. In the current PCI era, there are several observational studies, with controversial results.13–16,19–22 A possible explanation for these discordant results lies in the different baseline characteristics of the ACS populations (eg, percentage of patients with LVEF ≤ 40%, HF, type of ACS, DM, hypertension, chronic kidney disease), together with the differences in invasive (PCI rate) and medical (DAPT, beta-blockers, and statin use) management. In this regard, Gunnel et al.20 found no benefit in long-term mortality reduction by adding ACEI/ARB to medical therapy with beta-blockers and statins after an acute myocardial infarction treated with PCI. In our population, with all patients treated with PCI, and high rates of DAPT (> 90%), beta-blocker (> 80%), and statins (> 90%), we found a strong association of ACEI/ARB with lower 1-year mortality. However, our results differed according to LVEF. In patients with LVEF ≤ 40%, we found a substantial reduction in 1-year mortality with ACEI/ARB but not in patients with LVEF> 40%.
In ACS guidelines, ACEI/ARB are considered mandatory therapy in patients with LVEF ≤ 40%, with a class I recommendation and level of evidence A, unless contraindicated.1,2 This recommendation is based on 2 randomized clinical trials with postmyocardial infarction patients—the SAVE and TRACE trials8,9—with 2231 and 1749 patients with reduced LVEF (≤ 40% for SAVE and ≤ 35% for TRACE trial) randomized to captopril or trandolapril vs placebo, respectively. The reduction in the relative risk of death was 19% in the SAVE trial (average of follow-up 42 months) and 22% in the TRACE trial (average follow-up 37 months), and these results are similar to those of HF trials with ACEI/ARB and reduced LVEF, such as the SOLVD and CHARM trials.11,12 However, in both trials, the percentage of patients treated with antiplatelet therapy was <60% and that treated with beta-blockers was <40%; there were no data about statin prescription, and only <20% of patients underwent PCI. Our results extrapolate the benefit found in these 2 trials from the thrombolysis era to the contemporary PCI era, in a population treated according to current recommendations.
In patients with LVEF> 40%, we found no overall or consistent benefit of ACEI/ARB. Although most of the adjusted analyses were not significant in those patients, the instrumental variable analysis showed a benefit of ACEI/ARB. This suggests that, in post-ACS patients with LVEF> 40%, it is not clear whether ACEI/ARB have a prognostic benefit in reducing mortality or not. Given the heterogeneity of this group of patients, prescription should be individualized according to each patient's characteristics. When we analyzed subgroups in patients with post-ACS LVEF> 40%, we observed a significant reduction in 1-year death risk only in STEMI patients, and a trend to lower mortality with ACEI/ARB in high-risk patients (with HF, renal failure, DM, or hypertension). To the best of our knowledge, there are no large specific studies that analyze the role of ACEI/ARB in ACS patients with LVEF> 40%, beyond a contemporary small study.16 Parashar et al.16 analyzed the prognostic impact of ACEI/ARB in AMI patients with LVEF> 40% treated with PCI and glomerular filtration rate> 60 mL/min/1.73 m2. These authors found no benefit in long-term mortality by using ACEI/ARB in patients undergoing primary PCI. In the setting of stable coronary artery disease with preserved LVEF, there are 3 well-powered clinical trials with contradictory results.23–25 The HOPE study showed a significant reduction in the 5-year death rate with ramipril.25 Similar results were reported for the EUROPA trial with perindopril at 4 years.23 In the PEACE trial,24 8290 patients with stable coronary artery disease and normal or near-normal LVEF were randomly assigned to receive trandolapril or placebo. ACEI therapy was not associated with a lower death rate at 4.8 years. To interpret the predominantly negative findings of this study in the context of the positive reports from both HOPE and EUROPA, it is useful to compare the patient characteristics and the event rates in those 2 trials with those in the PEACE trial. At baseline, the patients in the PEACE trial had an average LVEF of 58%, and their average creatinine and cholesterol concentrations were normal.24 Their average blood pressure at baseline was 133/78mmHg, which was the level achieved with use of an ACE inhibitor in both HOPE and EUROPA.23,25 The patients in the PEACE trial also received more intensive risk factor management than did those in HOPE and EUROPA. At baseline, 70% of the patients (compared with 29% in HOPE and 56% in EUROPA) were receiving lipid-lowering therapy. Moreover, 72% of the patients in the PEACE trial, compared with 54% in EUROPA and 40% in HOPE, had undergone coronary revascularization before enrollment.23–25 It seems reasonable that this more aggressive strategy might have contributed to the lower risk of adverse events in the PEACE trial. Therefore, it is not surprising that with more intensive treatment of coronary artery disease and risk factor modification, adverse cardiovascular outcomes in patients assigned to placebo were substantially lower in PEACE than they were in the other 2 trials.
European ACS guidelines recommend (also as class I recommendation and level of evidence) the use of ACEI/ARB–even if LVEF is> 40%–in ACS patients with DM, HF, and hypertension1,2; and American guidelines extended this recommendation to patients with stable chronic kidney disease. The recommendation of ACEI/ARB in STEMI patients is based on 3 clinical trials (GISSI-3 for lisinopril, ISIS-4 for catopril, and SMILE-1 for zofenopril,3,5,7 which showed a significant reduction in 6-week mortality (5 weeks in the ISIS-4 trial) with ACEI in patients with AMI (with and without LVEF> 40%). However, in these trials, there was no systematic use of PCI (0% in the SMILE trial, not reported in GISSI-3 or in ISIS-4, with> 70% of thrombolysis in both), with a very low rate of use beta-blockers (< 30%) and statins (< 10%).3,5,7 Similar to the PEACE trial, our results do not support the widespread use of ACEI/ARB in ACS patients with LVEF> 40%. However, we have specifically reported a benefit in STEMI patients with LVEF> 40%, and a possible benefit in high-risk patients with LVEF> 40%. In low-risk patients with NSTEACS and LVEF> 40%, we found no benefit of ACEI/ARB in reducing 1-year mortality. Nevertheless, most physicians worldwide continue to prescribe ACEI/ARB in all patients after ACS, often because of a tendency to therapeutic optimism. Based on our results and the controversial prior studies, we believe that patients with post-ACS LVEF ≥ 40% without high-risk conditions (no STEMI, no HF, no DM, no hypertension, no kidney disease) represent an interesting clinical scenario for the performance of a randomized clinical trial according to the current recommendations in ACS management (Figure 5).
Evidence of ACEI or ARB after ACS in patients who underwent percutaneous coronary intervention. ACEI, angiotensin- converting enzyme inhibitors; ACS, acute coronary syndrome; ARB, angiotensin receptor blockers; LVEF, left ventricular ejection fraction; NSTEACS, non–ST-segment elevation acute coronary syndrome; STEMI, ST-segment elevation myocardial infarction.
This study has some limitations. First, only patients who survived hospital stay were studied and, consequently, we did not investigate the role of in-hospital ACEI/ARB. Several investigators in the thrombolysis era have shown that the benefit of ACEI/ARB occurs during the first few days after ACS, suggesting that mechanisms other than benefits on the remodeling process may play a role.3,5,7 Second, the survival benefits of ACEI/ARB were compared based on medications at discharge. In addition, the prescription dose, long-term adherence, discontinuation, incidence of adverse events, and drug information after discharge were not available in the present study. Moreover, we do not have data about prior use of ACEI/ARB before hospital admission. Third, we did not differentiate between ACEI and ARB. Although several authors have shown that both ACEI and ARB show an equal benefit, other authors have reported marked differences between the 2 drugs,26,27 showing different long-term tolerance and adherence.28 Fourth, our study included a selected and nonrandomized sample; in addition, although propensity scoring and instrumental variable analysis were adjusted for confounding by indication, and further adjustments were made for many additional confounders in the survival models, residual confounding is probable.
CONCLUSIONSThe benefit of ACEI/ARB in post-ACS patients is centered on those with LVEF ≤ 40% and STEMI. In NSTEMI patients with LVEF> 40%, further contemporary studies are needed to assess the long-term impact of ACEI or ARB in the modern PCI era in addition to other guideline-recommended cardiovascular drugs.
CONFLICTS OF INTERESTNone declared.
- –
ACEI and ARB have shown a benefit in reducing mortality after an ACS in patients with LVEF ≤ 40%. However, the benefit of these 2 therapies in ACS patients with LVEF> 40% who have undergone percutaneous coronary revascularization has not been tested in clinical trials. Nevertheless, they are still recommended by clinical practice guidelines for patients with hypertension, diabetes, or renal dysfunction.
- –
After an ACS treated with percutaneous coronary intervention, ACEI/ARB reduced mortality in patients with LVEF ≤ 40%.
- –
In ACS patients with LVEF> 40%, the benefit of ACEI was limited to STEMI patients.
- –
In NSTEACS with LVEF> 40%, ACEI/ARB could be beneficial in patients with hypertension, diabetes, chronic kidney diseas, or HF.
- –
Further contemporary studies are needed to assess the long-term impact of ACEI and ARB in the modern era of percutaneous coronary revascularization.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2019.02.012.