To the Editor,
Microalbuminuria cannot be ignored by cardiologists because it is considered a predictor of coronary artery disease in patients with type 2 diabetes. Angiotensin II receptor blockers (ARB-II) have been accepted nephroprotective agents in patients with type 2 diabetes with microalbuminuria since publication of the Irbesartan Patients with Diabetes and Microalbuminuria (IRMA-2) study.1 In patients with macroalbuminuria, the Reduction of Endpoints in NIDDM with Angiotensin II Antagonist Losartan (RENAAL)2 and Irbesartan in Diabetic Nephropathy Trial (IDNT)3 studies showed a slowing of progression to terminal kidney disease. However, in patients with diabetes with microalbuminuria, the Diabetic Retinopathy Candesartan Trial (DIRECT)4 showed no significant reduction in microalbuminuria.
Recently, the Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study5 has been published. Interestingly, it found that the use of olmesartan vs placebo to be associated with a significantly reduced incidence of microalbuminuria (23% relative reduction). However, it also showed increased incidence of cardiovascular death with olmesartan (15 vs 3 patients; P=.01), mainly due to sudden cardiac death (7 patients vs 1) and death from myocardial infarction (5 patients vs 0). Any-cause mortality was unfavorable, but non-significant, for olmesartan (26 vs 15 patients).
In an attempt to clarify this recent concern, we aimed to determine the safety in terms of mortality of ARB-II use in patients with type 2 diabetes with normoalbuminuria, microalbuminuria or macroalbuminuria in a combined analysis.
The present meta-analysis included all randomized placebo-controlled studies of patients with type 2 diabetes and using ARB-II in the intervention group, published in English- or Spanish-language peer-review journals that present mortality data (at least any-cause mortality). We conducted a systematic review of MEDLINE/PubMed and ISI Web-of-Knowledge databases until April 2011. The search terms were losartan, irbesartan, valsartan, olmesartan, candesartan, eprosartan, telmisartan, combined with diabetic nephropathy and randomized trial. We also reviewed meta-analyses and recent review articles.
We calculated the relative risk (RR) with 95% confidence interval using Mantel-Haenszel weighting. We determined heterogeneity using Cochran's Q test and the H- and I-statistics.2 Publication bias was determined using the Egger and Macaskill method. We also performed an analysis of sensitivity. Statistical analysis was with SPSS 15 and the Domenech JM macro (Macro!MAR for SPSS Statistics, V2010.04.15. UAB).
Of 459 articles, only five met our inclusion criteria1, 2, 3, 4, 5 (1.1%); these included 9603 patients (Table 1). Essentially, the causes of exclusion were: a) nonrandomized design; b) lack of placebo group; c) lack of data on mortality, and d) “non-informative” studies (0 mortal events in intervention and control groups). Except for Haller5, the remaining articles1, 2, 3, 4 reported no specific individualized data on “cardiovascular-cause” mortality.
Table 1. Baseline Characteristics of Patients With Type 2 Diabetes in Each of the Studies Chosen (in the Placebo Group)
| Brenner et al. 2 | Parving et al. 1 | Lewis et al. 3 | Bilous et al. 4 | Haller et al. 5 | |
| Name of study/group | RENAAL | IRMA-2 | IDNT | DIRECT-Protect2 | ROADMAP |
| Year of publication | 2001 | 2001 | 2001 | 2009 | 2011 |
| ARB-II studied | Losartan | Irbesartan | Irbesartan | Candesartan | Olmesartan |
| Total sample size a | 1513 | 590 | 1148 | 1905 | 4447 |
| Age, years | 60±7 | 58.3±8.7 | 58.3±8.2 | 56.8±7.9 | 57.8±8.6 |
| Caucasian, % | 49.6 | 98 | 78 | 96 | 100 |
| Men, % | 64.8 | 68.7 | 71 | 51 | 45.3 |
| BMI | 29±6 | 30.3±4.4 | 30.5±5.9 | 29.4±4.8 | 30.9±4.9 |
| Coronary disease, % | 22.1 | 4.5 | 29 b | NR | 24.4 |
| Myocardial infarction, % | 12.3 | 1.5 | NR | NR | 5.4 |
| Stroke or TIA | 0.1 | 3.5 | NR | NR | 2.2 |
| Peripheral arterial disease, % | NR | 4 | NR | NR | 0.4 |
| Creatinine, μmol/l | 168±44.2 | 88.4±8.8 | 149.4±50.4 | 90.1±15.2 | 77.5±17.1 |
| Glycohemoglobin, % | 8.4±1.6 | 7.1±1.6 | 8.2±1.7 | 8.2±1.6 | 7.7±1.6 |
| Albuminuria a | Macroalbuminuria | Microalbuminuria | Macroalbuminuria | Normoalbuminuria | Normoalbuminuria |
| High blood pressure at enrolment a | Yes | Yes | Yes | 62% | NR |
| Mean follow-up, years a | 3.4 | 2 | 2.6 | 4.7 | 3.2 |
| Mortality intervention group, n/N | 158/751 | 3/389 | 87/579 | 37/949 | 26/2232 |
| Mortality placebo group, n/N | 155/762 | 1/201 | 93/569 | 35/953 | 15/2215 |
ARB-II, angiotensin II receptor blockers; BMI, body mass index; NR, not reported; TIA, transitory ischemic accident.
a Intervention group with each ARB-II and non-intervention (placebo) group.
b Described in the original publication as “history of cardiovascular disease”.Continuous variables are expressed as mean± standard deviation and categorical variables as percentage.
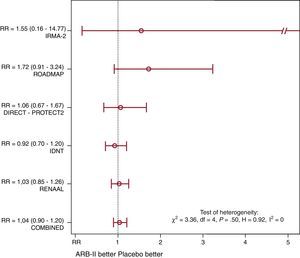
Of 4900 patients in the group receiving ARB-II, 311 (6.3%) died during follow-up (of whatever cause) vs 299 of 4700 (6.4%) in the placebo group (Mantel-Haenszel RR=1.04; P=.61) (Figure 1). Although the studies with lower baseline risk (ROADMAP5 and IRMA-21) tended to present a less favorable RR in patients receiving ARB-II, there was no evidence of significant heterogeneity. Sensitivity analysis was concordant and we found no publication bias.
Figure 1. Comparative effect of angiotensin II receptor blockers in patients with type 2 diabetes with respect to any-cause death (expressed as relative risk). ARB-II, angiotensin II receptor blockers; RR, relative risk.
The unexpected ROADMAP study5 findings on increased cardiovascular-cause mortality in patients receiving olmesartan conflicts with recent trials, principally, the ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET)6 that included 9612 patients with diabetes and reported telmisartan had a beneficial effect similar to that of ramipril—which had previously been shown to reduce myocardial infarction and mortality. It has been argued that some of the excess events occurred in patients with previous ischemic heart disease and in patients in whom olmesartan induced a minimum hypotensive effect or a substantial reduction in blood pressure, raising once more the controversial “J curve” issue with respect to mortality and ischemic heart disease.5, 6
We conclude that although previous studies have adequately shown that ARB-II use slows kidney disease in a range of patients with type 2 diabetes with different degrees of vascular disease, the present meta-analysis shows no benefits but nor does it show any harm to patients’ global survival. Further studies will need to determine whether the conflictive ROADMAP study5 results are a cause for concern because they reflect a specific effect of olmesartan—which cannot be discounted—or simply a chance finding. This raises the interesting question as to whether microalbuminuria remains a good intermediate variable to predict cardiovascular outcomes.
.
Corresponding author. lconsue@gmail.com