Transvenous pacemakers are contraindicated in Eisenmenger syndrome due to the high risk of paradoxical embolism. Leadless pacemakers are preferred because of their tendency to become encapsulated and the absence of leads in low-pressure chambers (right atrium [RA]).
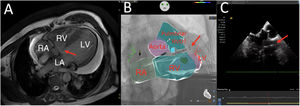
We present the case of a 71-year-old man admitted for complete atrioventricular block. The patient had uncorrected congenital heart disease with a double-chambered right ventricle (RV) and a perimembranous interventricular septal defect with bidirectional flow, pulmonary hypertension in the context of Eisenmenger syndrome, and moderate chronic pericardial effusion (figure 1A, arrow; LA, left atrium; LV, left ventricle). Considering the patient's history of documented paroxysmal atrial fibrillation, it was decided to implant a leadless pacemaker.
The pacemaker was deployed in the right ventricular outflow tract (RVOT) with the aid of intracardiac echocardiography-fluoroscopy fusion imaging (Cartosound-Univu electroanatomic navigation modules; Biosense Webster, USA). This approach circumvented the need to access the left cavities through the ventricular septal defect, enabled exclusion of the hypertrabeculated part of the RV, and guided the selection of an optimal implantation site.
Excellent pacing and sensing parameters were observed following the initial delivery of the pacemaker into the RVOT ().
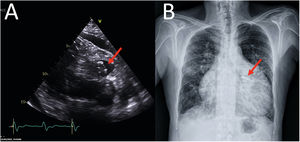
The final position of the device is shown in Figure 2A, B (echocardiogram and chest radiograph, respectively) and . Informed consent was obtained from the patient for publication of case details.
Leadless pacemakers could be the best option for patients with Eisenmenger syndrome due to their lower thrombogenicity. Hybrid imaging techniques potentially improve the safety of pacemaker implantation.
FundingNone
Authors’ ContributionsAll the authors wrote, reviewed, and approved the manuscript.
Conflicts of InterestJ.L. Martínez-Sande has received consultancy fees from Medtronic. The other authors declare that they have no conflicts of interest.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.08.010