In recent years, sodium-glucose cotransporter 2 inhibitors (SGLT2i) have demonstrated significant heart failure (HF) benefits in patients with HF with reduced ejection fraction (HFrEF) regardless of the presence of type 2 diabetes (T2D).1 Glucagon-like peptide-1 receptor agonists (GLP-1ra) have also been shown to significantly reduce hospitalizations for HF in patients with T2D in a meta-analysis of pivotal cardiovascular clinical trials, although these benefits were not achieved in each individual clinical trial.2 Furthermore, the evidence provided by observational studies is fairly limited and controversial.3
We performed a prospective, multicenter, real-world study in patients with T2D and HFrEF treated with semaglutide (Sema-Reduced-Group) and without semaglutide or another GLP-1ra (control-reduced-group) and followed up for 52 weeks between June 2019 and May 2023.
The diagnosis of HFrEF was established according to the 2021 European Society of Cardiology Guidelines.4
Data on multiple clinical variables were gathered at each evaluation.
The primary outcome was the number of HF events (a composite of emergency department visits, hospitalizations, and unscheduled outpatient visits). Secondary outcomes included the individual components of the primary outcome, cardiovascular death, all-cause death, all-cause hospitalizations, new or worsening nephropathy, and ≥ 5-point difference in the Kansas City Cardiomyopathy Questionnaire (KCCQ) total symptom score change from baseline to 52 weeks of follow-up.
To match each patient in each group in a 1:1 manner, we used propensity score matching (PSM). The adequacy of the PSM was assessed using the standardized difference (a significant imbalance was considered with a standardized difference >10% between baseline variables). The probability of starting semaglutide was estimated using a logistic regression model. The Pearson correlation coefficient was calculated to estimate linear correlations. To evaluate the association between treatment and study outcomes, mixed effect logistic regressions were used and adjusted for confounding variables.
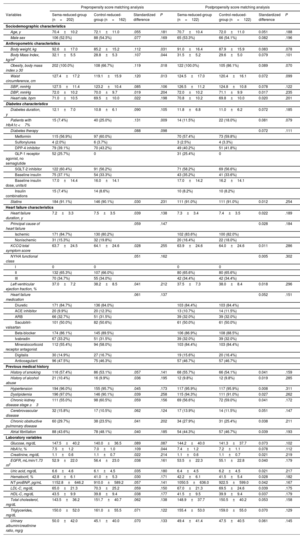
A total of 202 patients were included in the sema-reduced-group, and 162 patients in the control-reduced-group. After PSM, 122 patients were included in each group. At 52 weeks, 104 patients (85.2%) had received 1.00mg of once weekly semaglutide. Baseline characteristics are shown in table 1.
Sociodemographic, clinical, and therapeutic characteristics at baseline: pre- and postpropensity score matching analysis
| Prepropensity score matching analysis | Postpropensity score matching analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Sema-reduced-group (n=202) | Control-reduced-group (n=162) | Standardized difference | P | Sema-reduced-group (n=122) | Control-reduced-group (n=122) | Standardized difference | P |
| Sociodemographic characteristics | ||||||||
| Age, y | 70.4±10.2 | 72.1±11.0 | .055 | .181 | 70.7±10.4 | 72.0±11.0 | 0.051 | .188 |
| Male sex | 106 (52.5%) | 88 (54.3%) | .077 | .169 | 65 (53.3%) | 66 (54.1%) | 0.082 | .196 |
| Anthropometric characteristics | ||||||||
| Body weight, kg | 92.6±17.0 | 85.2±15.2 | .112 | .031 | 91.0±16.4 | 87.9±15.9 | 0.083 | .078 |
| Body Mass Index, kg/m2 | 32.1±5.5 | 28.8±5.3 | .107 | .044 | 31.5±5.2 | 29.6±5.0 | 0.079 | .101 |
| Obesity, body mass index ≥ 30 | 202 (100.0%) | 108 (66.7%) | .119 | .018 | 122 (100.0%) | 105 (86.1%) | 0.089 | .070 |
| Waist circumference, cm | 127.4±17.2 | 119.1±15.9 | .120 | .013 | 124.5±17.0 | 120.4±16.1 | 0.072 | .099 |
| SBP, mmHg | 127.5±11.4 | 123.2±10.4 | .085 | .106 | 126.5±11.2 | 124.8±10.8 | 0.078 | .122 |
| DBP, mmHg | 72.0±10.2 | 70.0±9.7 | .019 | .204 | 72.0±10.2 | 71.1±9.9 | 0.017 | .235 |
| Heart rate, bpm | 71.0±10.5 | 69.5±10.0 | .022 | .198 | 70.8±10.2 | 69.8±10.0 | 0.020 | .201 |
| Diabetes characteristics | ||||||||
| Diabetes duration, y | 12.1±7.0 | 10.8±6.1 | .090 | .105 | 11.8±6.8 | 11.0±6.2 | 0.072 | .185 |
| Patients with HbA1c <7% | 15 (7.4%) | 40 (25.0%) | .131 | .009 | 14 (11.5%) | 22 (18.0%) | 0.081 | .079 |
| Diabetes therapy | .088 | .098 | 0.072 | .111 | ||||
| Metformin | 115 (56.9%) | 97 (60.0%) | 70 (57.4%) | 73 (59.8%) | ||||
| Sulfonylurea | 4 (2.0%) | 6 (3.7%) | 3 (2.5%) | 4 (3.3%) | ||||
| DPP-4 inhibitor | 79 (39.1%) | 70 (43.2%) | 49 (40.2%) | 51 (41.8%) | ||||
| GLP-1 receptor agonist, no semaglutide | 52 (25.7%) | 0 | 31 (25.4%) | 0 | ||||
| SGLT-2 inhibitor | 122 (60.4%) | 91 (56.2%) | 71 (58.2%) | 69 (56.6%) | ||||
| Baseline insulin | 75 (37.1%) | 54 (33.3%) | 43 (35.2%) | 41 (33.6%) | ||||
| Baseline insulin dose, units/d | 17.0±14.4 | 16.0±14.1 | 17.0±14.2 | 16.2±14.1 | ||||
| Insulin combinations | 15 (7.4%) | 14 (8.6%) | 10 (8.2%) | 10 (8.2%) | ||||
| Statins | 184 (91.1%) | 146 (90.1%) | .030 | .231 | 111 (91.0%) | 111 (91.0%) | 0.012 | .254 |
| Heart failure characteristics | ||||||||
| Heart failure duration, y | 7.2±3.3 | 7.5±3.5 | .039 | .138 | 7.3±3.4 | 7.4±3.5 | 0.022 | .189 |
| Principal cause of heart failure | .059 | .147 | 0.028 | .184 | ||||
| Ischemic | 171 (84.7%) | 130 (80.2%) | 102 (83.6%) | 100 (82.0%) | ||||
| Nonischemic | 31 (15.3%) | 32 (19.8%) | 20 (16.4%) | 22 (18.0%) | ||||
| KCCQ total symptom score | 63.7±24.5 | 64.1±24.6 | .028 | .255 | 63.9±24.6 | 64.0±24.6 | 0.011 | .286 |
| NYHA functional class | .051 | .162 | 0.005 | .302 | ||||
| I | 0 | 0 | 0 | 0 | ||||
| II | 132 (65.3%) | 107 (66.0%) | 80 (65.6%) | 80 (65.6%) | ||||
| III | 70 (34.7%) | 55 (34.0%) | 42 (34.4%) | 42 (34.4%) | ||||
| Left ventricular ejection fraction, % | 37.0±7.2 | 38.2±8.5 | .041 | .212 | 37.5±7.3 | 38.0±8.4 | 0.018 | .296 |
| Heart failure medication | .061 | .137 | 0.052 | .151 | ||||
| Diuretic | 171 (84.7%) | 136 (84.0%) | 103 (84.4%) | 103 (84.4%) | ||||
| ACE inhibitor | 20 (9.9%) | 20 (12.3%) | 13 (10.7%) | 14 (11.5%) | ||||
| ARB | 66 (32.7%) | 51 (31.5%) | 39 (32.0%) | 39 (32.0%) | ||||
| Sacubitril-valsartan | 101 (50.0%) | 82 (50.6%) | 61 (50.0%) | 61 (50.0%) | ||||
| Beta-blocker | 174 (86.1%) | 145 (89.5%) | 106 (86.9%) | 108 (88.5%) | ||||
| Ivabradin | 67 (33.2%) | 51 (31.5%) | 39 (32.0%) | 39 (32.0%) | ||||
| Mineralocorticoid receptor antagonist | 112 (55.4%) | 94 (58.0%) | 103 (84.4%) | 103 (84.4%) | ||||
| Digitalis | 30 (14.9%) | 27 (16.7%) | 19 (15.6%) | 20 (16.4%) | ||||
| Anticoagulant | 96 (47.5%) | 75 (46.3%) | 57 (46.7%) | 57 (46.7%) | ||||
| Previous medical history | ||||||||
| History of smoking | 116 (57.4%) | 86 (53.1%) | .057 | .141 | 68 (55.7%) | 66 (54.1%) | 0.041 | .159 |
| History of alcohol abuse | 21 (10.4%) | 16 (9.9%) | .036 | .195 | 12 (9.8%) | 12 (9.8%) | 0.019 | .285 |
| Hypertension | 194 (96.0%) | 155 (95.7%) | .045 | .173 | 117 (95.9%) | 117 (95.9%) | 0.008 | .311 |
| Dyslipidemia | 196 (97.0%) | 146 (90.1%) | .039 | .258 | 115 (94.3%) | 111 (91.0%) | 0.027 | .262 |
| Chronic kidney disease stage ≥3 | 111 (55.0%) | 98 (60.5%) | .059 | .156 | 69 (56.6%) | 72 (59.0%) | 0.041 | .172 |
| Cerebrovascular disease | 32 (15.8%) | 17 (10.5%) | .062 | .124 | 17 (13.9%) | 14 (11.5%) | 0.051 | .147 |
| Chronic obstructive pulmonary disease | 60 (29.7%) | 38 (23.5%) | .041 | .202 | 34 (27.9%) | 31 (25.4%) | 0.038 | .211 |
| Atrial fibrillation | 88 (43.6%) | 78 (48.1%) | .040 | .185 | 54 (44.3%) | 57 (46.7%) | 0.039 | .193 |
| Laboratory variables | ||||||||
| Glucose, mg/dL | 147.5±40.2 | 140.0±36.5 | .089 | .087 | 144.2±40.0 | 141.3±37.7 | 0.073 | .102 |
| HbA1c, % | 7.5±1.2 | 7.0±1.0 | .109 | .044 | 7.4±1.2 | 7.2±1.1 | 0.078 | .112 |
| Creatinine, mg/dL | 1.1±0.6 | 1.1±0.7 | .022 | .214 | 1.1±0.6 | 1.1±0.7 | 0.021 | .219 |
| EGFR, mL/min/1.73 m2 | 52.9±22.0 | 56.4±23.0 | .038 | .161 | 53.5±22.3 | 55.1±22.8 | 0.040 | .179 |
| Uric acid, mg/dL | 6.6±4.6 | 6.1±4.5 | .035 | .180 | 6.4±4.5 | 6.2±4.5 | 0.021 | .217 |
| Hematocrit, % | 42.8±6.1 | 41.0±5.3 | .030 | .171 | 42.2±6.1 | 41.5±5.4 | 0.028 | .182 |
| NT-proBNP, pg/mL | 1152.8±646.2 | 910.0±589.2 | .057 | .141 | 1050.5±636.0 | 922.5±599.0 | 0.042 | .167 |
| LDL-C, mg/dL | 65.0±21.3 | 70.3±25.2 | .059 | .150 | 67.0±21.3 | 69.5±24.6 | 0.039 | .175 |
| HDL-C, mg/dL | 43.5±9.9 | 39.8±9.4 | .038 | .177 | 41.5±9.5 | 39.9±9.4 | 0.037 | .179 |
| Total cholesterol, mg/dL | 143.5±36.2 | 151.7±40.7 | .062 | .138 | 148.9±37.7 | 150.5±40.2 | 0.053 | .158 |
| Triglycerides, mg/dL | 150.0±52.0 | 161.0±55.5 | .071 | .122 | 155.4±53.0 | 159.0±55.0 | 0.070 | .129 |
| Urinary albumin/creatinine ratio, mg/g | 50.0±42.0 | 45.1±40.0 | .070 | .133 | 49.4±41.4 | 47.5±40.5 | 0.061 | .145 |
Continuous data are shown as means±standard deviation and qualitative data as No. (%). The differences between groups were determined using the 2-sample Student t-test or the Mann-Whitney-Wilcoxon rank-sum test for continuous variables and Pearson's chi-square for categorical variables.
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; DBP, diastolic blood pressure; DPP4, dipeptidyl peptidase-4; EGFR, estimated glomerular filtration rate; GLP-1, glucagon-like peptide-1; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; LDL-C, low-density lipoprotein cholesterol; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SGLT-2, sodium-glucose cotransporter 2.
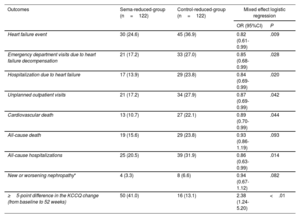
Once weekly semaglutide was associated with reductions in HF events and their individual components. Furthermore, cardiovascular death and all-cause hospitalizations decreased significantly. Last, patients in the sema-reduced-group were more likely to have a ≥ 5-point difference on the KCCQ score change (improving 18.7±3.2 vs 8.2±1.7 points (P<.01) compared with the control-reduced-group. Outcomes results are shown in table 2.
Primary and secondary outcomes
| Outcomes | Sema-reduced-group (n=122) | Control-reduced-group (n=122) | Mixed effect logistic regression | |
|---|---|---|---|---|
| OR (95%CI) | P | |||
| Heart failure event | 30 (24.6) | 45 (36.9) | 0.82 (0.61-0.99) | .009 |
| Emergency department visits due to heart failure decompensation | 21 (17.2) | 33 (27.0) | 0.85 (0.68-0.99) | .028 |
| Hospitalization due to heart failure | 17 (13.9) | 29 (23.8) | 0.84 (0.69-0.99) | .020 |
| Unplanned outpatient visits | 21 (17.2) | 34 (27.9) | 0.87 (0.69-0.99) | .042 |
| Cardiovascular death | 13 (10.7) | 27 (22.1) | 0.89 (0.70-0.99) | .044 |
| All-cause death | 19 (15.6) | 29 (23.8) | 0.93 (0.86-1.19) | .093 |
| All-cause hospitalizations | 25 (20.5) | 39 (31.9) | 0.86 (0.63-0.99) | .014 |
| New or worsening nephropathy* | 4 (3.3) | 8 (6.6) | 0.94 (0.67-1.12) | .082 |
| ≥5-point difference in the KCCQ change (from baseline to 52 weeks) | 50 (41.0) | 16 (13.1) | 2.38 (1.24-5.20) | <.01 |
Data are shown as No. (%). To evaluate the association between treatment and study outcomes, mixed effect logistic regressions were used. The regression analysis values are expressed as odds ratio and 95% confidence interval. Values were considered statistically significant if P<.05.
95%CI, 95% confidence interval; KCCQ, Kansas City Cardiomyopathy Questionnaire; OR, odds ratio.
Patients treated with semaglutide had a larger reduction in HbA1c (0.9±0.2 vs 0.3±0.1%, P=.011) and body weight (11.8±3.8 vs 2.5±1.1kg, P<.01) than those in the control-reduced-group. There were negative correlations between the KCCQ score and HbA1c (r=0.532, P<.009) and body weight (r=–0.649, P<.01).
Regarding safety, fewer serious adverse events occurred among patients who received semaglutide (24.6%). Adverse events were mostly gastrointestinal, and 11 patients (9.0%) discontinued the drug.
While SGLT2i have shown benefits in HF outcomes in patients with HF with and without T2D, GLP-1ra have not been strongly associated with reductions in HF hospitalizations.2 The evidence from observational studies is fairly limited and controversial, being associated with neutral effects in some studies and beneficial effects, namely a reduction in HF hospitalizations, in other studies.3 Recently, once weekly semaglutide has been associated with cardiovascular and HF benefits in overweight/obese patients with pre-existing cardiovascular disease,5 and HF with preserved ejection fraction.6 The HF benefits of GLP-1ra could be achieved through multiple interrelated mechanism such as the direct effects on endothelium, cardiac tissue, renin-angiotensin system, and cardiometabolic risk factors.3,5
The above-mentioned benefits on HF outcomes found in several studies (some including patients with HFrEF with coronary artery and cerebrovascular diseases) are consistent with our results. The implementation of structured treatment programs including the use of GLP-1ra in combination with improvements in the quality of diet and exercise to achieve long-term body weight loss and an increase in lean mass could be established as an important goal in the management of patients with T2D, overweight/obesity, and HFrEF.
Although these findings provide valuable information, they should be considered within the context of potential limitations such as the possibility of unmeasured confounding factors, the relatively low number of some outcomes, and the influence of changes in HF treatment and general recommendations made during the follow-up.
In conclusion, in this observational study and after a PSM, once weekly semaglutide was associated with a reduction in HF events, cardiovascular death, and all-cause hospitalizations in patients with T2D and HFrEF. Furthermore, patients treated with semaglutide were more likely to have a ≥ 5-point difference on the KCCQ total symptom score from baseline to 52 weeks. Further research is needed on GLP-1ra in HFrEF.
FUNDINGThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ETHICAL CONSIDERATIONSThe study was approved by the Institutional Research Ethics Committee of Málaga (Ethics Committee code: REDIME-27-10-2016) and written informed consent for the consultation of medical records was obtained from all participants. This study was conducted in accordance with the Declaration of Helsinki. Data confidentiality and patient anonymity were rigorously maintained during the performance of the study.
Gender disaggregation was not performed based on the outcomes of this study.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence was used in the preparation of this article.
AUTHORS’ CONTRIBUTIONSM.A. Pérez-Velasco: analysis and interpretation of data and manuscript preparation. A. Trenas: analysis and interpretation of data and manuscript preparation. M.R. Bernal-López: analysis and interpretation of data and manuscript preparation. M.D. García de Lucas: analysis and interpretation of data and manuscript preparation. R. Gómez-Huelgas: concept and design, analysis and interpretation of data, and manuscript preparation. L.M. Pérez-Belmonte: concept and design, acquisition of participants and data, analysis and interpretation of data, and manuscript preparation. All authors have participated in drafting the manuscript and have read and approved the final version of the manuscript. M.A. Pérez-Velasco and A. Trenas contributed equally to this work and share first authorship.
CONFLICTS OF INTERESTNone.