Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary syndrome, the incidence of which is increasing, probably due to increased diagnostic suspicion.1,2 The optimal treatment remains under debate, although there is a consensus on initial conservative treatment based on long-term prospective series.1,2 However, in cases of persistent ischemia, percutaneous coronary intervention is recommended, and in this situation the use of bioresorbable scaffolds (BRS) can be beneficial.3
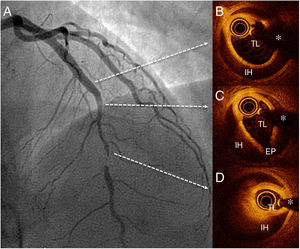
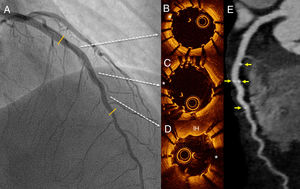
We present the case of a 47-year-old woman who presented with a 24-hour history of persistent chest pain, with no significant electrocardiographic changes. Blood tests showed elevated markers of myocardial damage (cardiac troponin I, 3.5μg/L), and echocardiography ruled out regional wall motion abnormalities. The patient's symptoms resolved and she was admitted with suspected acute myopericarditis. On day 5, she developed new chest pain, this time with ST-segment depression of up to 4mm in the anterior leads. Urgent transradial coronary angiography showed a long, severe lesion in the mid segment of the left anterior descending artery (figure 1 and ). The patient did not improve with intracoronary glyceryl trinitrate, and given the high suspicion of SCAD, optical coherence tomography (OCT) was performed. OCT confirmed the diagnosis of SCAD and revealed an intimal flap and a long (38mm) segment of intramural hematoma that was severely compressing the true lumen (minimal lumen area, 0.7mm2) (figure 1B-D and ). Due to the persistence of chest pain and poor hemodynamic tolerance, it was decided to perform percutaneous coronary intervention with fenestration with a scoring balloon catheter (Angiosculpt, 2.5×15 mm) with OCT guidance to try to decompress the false lumen. After several dilatations, the distal flow improved, but the luminal compromise persisted, and 2 overlapping magnesium BRS were placed (Magmaris, 3.0×2.0 mm distally and 3.5×15 mm proximally), with a good final result (figure 2A and ). Follow-up OCT showed good apposition of the 2 BRS and good compression of the intramural hematoma; an image of minimal intimal rupture was seen proximal to the treated segment (figure 2B-D and ). Computed tomography at 72hours showed an excellent result with the BRS and confirmed no progression of the SCAD (figure 2E). The patient remained symptom-free and was discharged with clinical and computed tomography follow-up at 6 months.
SCAD causes a not insignificant proportion of acute coronary syndromes in women younger than 60 years; a high clinical suspicion is essential for the diagnosis.1,2 There is consensus on initial conservative treatment, given that in most cases, spontaneous resolution of the SCAD is observed on imaging after a few months.1,2 For patients with refractory or recurrent ischemia, percutaneous coronary intervention should be considered, although the best revascularization strategy remains to be established. Some authors advocate the use of scoring balloon catheters to fenestrate and decompress the subintimal hematoma and thus improve the results of stenting.4 There are some small series of patients with SCAD treated with first-generation BRS with good outcomes at follow-up. This strategy favors the possibility of complete vascular recovery and avoids the problem of late malapposition of the device after resorption of the intramural hematoma and the risk of late thrombosis.3 In the present case, the use of a scoring balloon did not achieve an adequate angiographic result, probably because we used an under-sized device (to avoid excessive insult to the damaged arterial wall), and ultimately we had to use a BRS. Due to the withdrawal of the first-generation BRS, we opted for a magnesium BRS and the choice of size was guided by OCT to accommodate the lamina elastica externa distal to the affected segment. After implanting the first BRS, we confirmed the persistence of hematoma in the proximal uncovered section, so it was necessary to overlap a second magnesium BRS.
The use of magnesium BRS has been described in 1 patient with SCAD, with a good outcome at 12 months on angiography and OCT follow-up.5 The rapid resorption time of this device (an estimated 12 months) makes it a particularly attractive option in this context. To the best of our knowledge, this is the first described case that shows the value of a combined strategy (fenestration and magnesium BRS) with a good final result confirmed on angiography, OCT, and computed tomography. Use of computed tomography avoids the need for further invasive procedures in these patients who have increased susceptibility to vascular complications. Furthermore, magnesium does not produce imaging artifacts on computed tomography, so this technique allows optimal visualization of the vascular lumen.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2019.06.015
Video 1 Angiography findings before percutaneous coronary intervention.
Video 2 Optical coherence tomography findings before percutaneous coronary intervention.
Video 3 Angiography findings after percutaneous coronary intervention.
Video 4 Optical coherence tomography findings after percutaneous coronary intervention.