In recent years, there has been a notable increase in the number of patients requiring surgery or an invasive diagnostic/therapeutic procedure while receiving chronic treatment with antithrombotic, antiplatelet, or anticoagulant medications.1 As there are few available randomized trials to provide guidance on the use of these agents in many scenarios, various consensus documents have been developed to aid in clinical decision-making.2–5 Among them, a Spanish document, promoted by the Cardiovascular Thrombosis Working Group of the Spanish Society of Cardiology and endorsed by more than 20 scientific societies,4 provides recommendations similar to those found in European guidelines for noncardiac surgery2 and in consensus documents from the United States, one of which is specific to interventional cardiology procedures.3,5 The REQXAA6 study, which evaluated adherence to antithrombotic therapy guidelines in Spain, concluded that compliance was deficient in 42.7% of cases. Furthermore, the incidence of severe thrombotic and bleeding complications at 30 days following the procedure was significantly higher in the group with inappropriate use of these medications.
This study included 1266 patients receiving antithrombotic therapy and undergoing surgery or other invasive procedures at various sites.6 Among the total, 288 patients (22.7%) underwent invasive cardiology procedures. The aim was to determine whether adherence to recommendations was higher in cardiology than in other medical settings, and to assess the potential impact of adherence on the prognosis and incidence of severe complications at postprocedure 30 days.
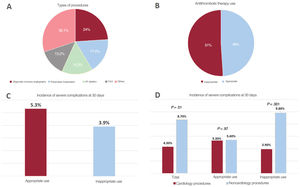
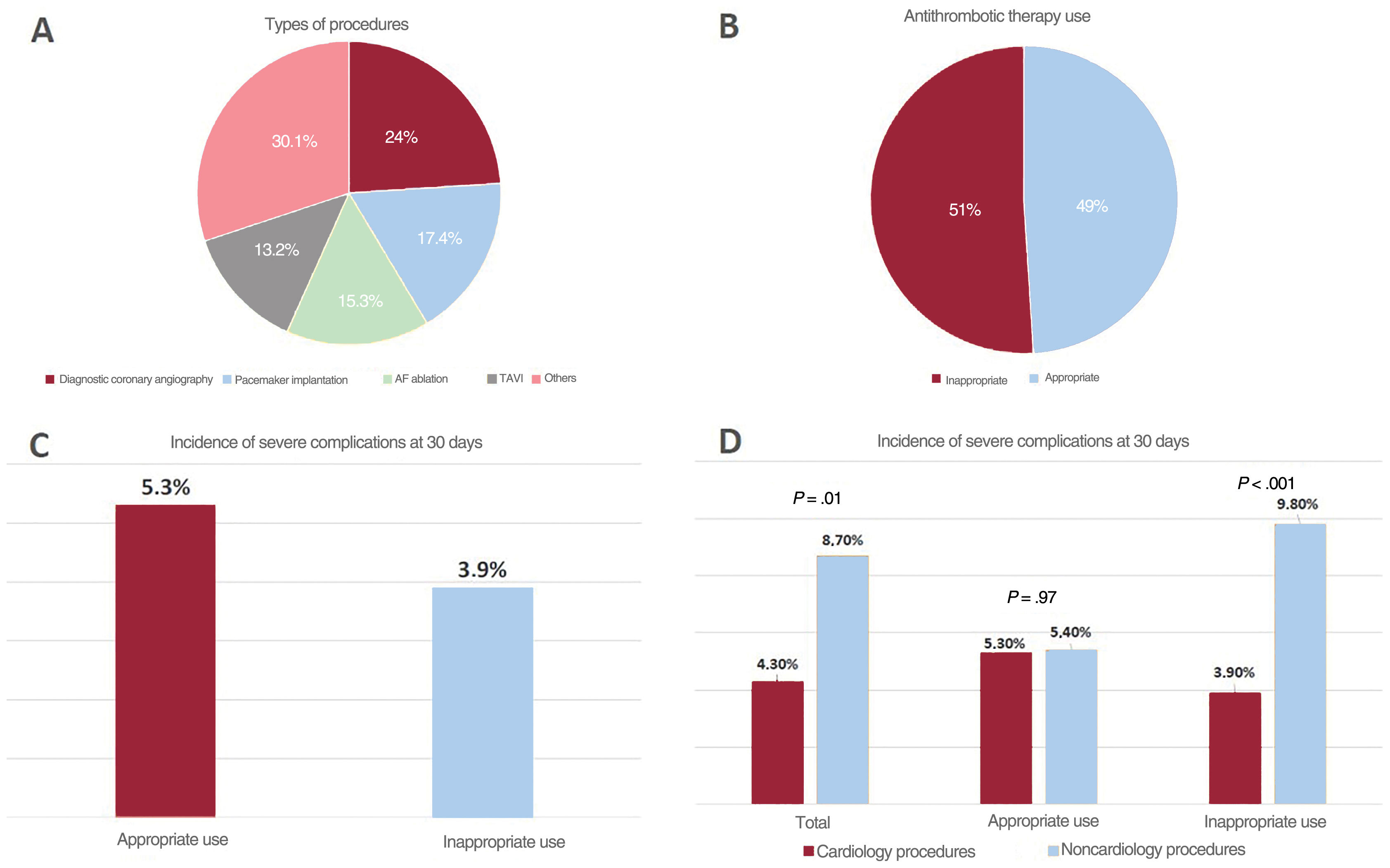
The study was approved by the Ethics Committee of Hospital Clínico San Carlos de Madrid. All patients provided signed informed consent. Among the 1266 patients included, 288 (22.7%) underwent an invasive cardiology procedure (figure 1A). There were no differences in age, sex, or the prevalence of hypertension or diabetes between this group and the noncardiology patients included (table 1). The prevalence of cardiac diseases (coronary artery disease, heart failure, and atrial fibrillation) was higher in the cardiology group, while extracardiac comorbidities were more common in the noncardiology group (table 1). Antiplatelet therapy was more prevalent in noncardiology patients, whereas anticoagulant use was more common in the cardiology group. In addition, a larger percentage of patients in the cardiology group had moderate to high thrombotic risk, whereas noncardiology patients showed a greater prevalence of moderate to high bleeding risk during the procedure.
A, Types of interventional cardiology procedures. B, Appropriate or inappropriate antithrombotic therapy use in cardiology procedures. C, Incidence of severe clinical events at 30 days following the procedure in groups with appropriate and inappropriate use of antithrombotic therapy. D, Comparison of the incidence of severe clinical events at 30 days between groups undergoing cardiology and noncardiology procedures (total, appropriate, and inappropriate antithrombotic therapy
AF, atrial fibrillation; TAVI, transcatheter aortic valve implantation.
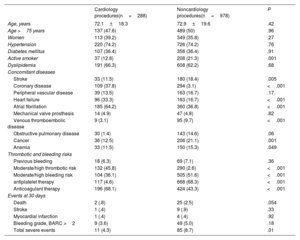
Comparison of clinical characteristics, treatment, comorbidities, and the incidence of events at 30 days between patients undergoing cardiology and noncardiology procedures
| Cardiology procedures(n=288) | Noncardiology procedures(n=978) | P | |
|---|---|---|---|
| Age, years | 72.1±18.3 | 72.9±19.6 | .42 |
| Age >75 years | 137 (47.6) | 489 (50) | .96 |
| Women | 113 (39.2) | 349 (35.8) | .27 |
| Hypertension | 220 (74.2) | 726 (74.2) | .76 |
| Diabetes mellitus | 107 (36.4) | 356 (36.4) | .91 |
| Active smoker | 37 (12.8) | 208 (21.3) | .001 |
| Dyslipidemia | 191 (66.3) | 608 (62.2) | .68 |
| Concomitant diseases | |||
| Stroke | 33 (11.5) | 180 (18.4) | .005 |
| Coronary disease | 109 (37.8) | 294 (3.1) | <.001 |
| Peripheral vascular disease | 39 (13.5) | 163 (16.7) | .17 |
| Heart failure | 96 (33.3) | 163 (16.7) | <.001 |
| Atrial fibrillation | 185 (64.2) | 360 (36.8) | <.001 |
| Mechanical valve prosthesis | 14 (4.9) | 47 (4.8) | .82 |
| Venous thromboembolic disease | 9 (3.1) | 95 (9.7) | <.001 |
| Obstructive pulmonary disease | 30 (1.4) | 143 (14.6) | .06 |
| Cancer | 36 (12.5) | 206 (21.1) | .001 |
| Anemia | 33 (11.5) | 150 (15.3) | .049 |
| Thrombotic and bleeding risks | |||
| Previous bleeding | 18 (6.3) | 69 (7.1) | .36 |
| Moderate/high thrombotic risk | 132 (45.8) | 290 (2.6) | <.001 |
| Moderate/high bleeding risk | 104 (36.1) | 505 (51.6) | <.001 |
| antiplatelet therapy | 117 (4.6) | 668 (68.3) | <.001 |
| Anticoagulant therapy | 196 (68.1) | 424 (43.3) | <.001 |
| Events at 30 days | |||
| Death | 2 (.8) | 25 (2.5) | .054 |
| Stroke | 1 (.4) | 9 (.9) | .33 |
| Myocardial infarction | 1 (.4) | 4 (.4) | .92 |
| Bleeding grade, BARC >2 | 9 (3.6) | 49 (5.0) | .18 |
| Total severe events | 11 (4.3) | 85 (8.7) | .01 |
Antithrombotic therapy use was inappropriate in 51% of cardiology procedures (figure 1B), compared to 40.2% of noncardiology interventions (p=.022). This suboptimal use mainly arose from discontinuation of medication that should have been maintained (62% of cases), with 15.6% of patients receiving bridging therapy with heparin. The total incidence of severe complications (death, myocardial infarction, stroke, pulmonary thromboembolism, major bleeding) at 30 days was 7.6% in the overall cohort, with a lower rate in the cardiology group (4.3% vs. 8.7%; p=.01) (table 1). Total mortality and the incidence of major bleeding events tended to be higher in the noncardiology group (table 1). Among patients undergoing cardiology procedures, the rate of severe complications was similar between those with appropriate and inappropriate drug use (5.3% vs 3.9%) (figure 1C). In cases of appropriate drug use, there were no significant differences in complication rates between the groups (5.3% vs 5.4%). However, among those with inappropriate drug use, cardiology patients showed significantly lower complication rates (3.9% vs 9.8%; p<.001) (figure 1D).
In conclusion, adherence to recommendations on periprocedural antithrombotic drug use seems to be poorer in cardiology than in other medical specialties, with a significantly higher rate of inappropriate use. However, this situation does not result in a less favorable prognosis for cardiology patients. These findings could be related to the low complication rate at 30 days, differences in the patient profiles, and the generally higher risk associated with surgical procedures (above all in emergency cases), all of which make direct comparisons challenging. Furthermore, recent guideline updates emerging after publication of the Spanish document may refine this conclusion, such as those advising against discontinuing anticoagulants for certain procedures (eg, pulmonary vein ablation).5 Therefore, broader, well-controlled studies are required to gain a better understanding of the clinical implications of these findings. Nonetheless, there is an evident need to address suboptimal adherence to relevant guideline recommendations among cardiologists. Implementation of targeted educational programs and strategies can help improve the clinical practice patterns within this specialty.
FUNDINGNone.
ETHICAL CONSIDERATIONSThe study was approved by the Ethics Committee of Hospital Clínico San Carlos de Madrid. All patients provided signed informed consent for participation. In this study, potential sex and gender variables have been considered in accordance with the SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence tools have been used in the preparation of this article.
AUTHORS’ CONTRIBUTIONSAll authors contributed equally to the design, writing, and revision of the manuscript.
CONFLICTS OF INTERESTD. Vivas: conferences for Daiichi Sankyo, AstraZeneca, Bayer, Pfizer, Abbott, Boehringer Ingelheim, Bristol-Myers-Squibb and Ferrer. R. Ferrandis: conferences for LFB, CSL Behring and Octapharma. M. Anguita Sánchez: conferences for Eli Lilly & Co, Daiichi Sankyo, AstraZeneca, Bayer, Pfizer, Boehringer Ingelheim, Bristol-Myers-Squibb and Novartis; consultancies for Eli Lilly & Co, Daiichi Sankyo, AstraZeneca, Bayer, Pfizer, Boehringer Ingelheim, Bristol-Myers-Squibb, and Novartis. F. Marín: conferences for AstraZeneca and Boehringer Ingelheim; consultancy for Boehringer Ingelheim; research grants from AstraZeneca, Ferrer, and BMS. The remaining authors have no conflicts of interest to declare.
The authors are grateful for the participation of all researchers involved in the REQXAA study (supplementary data).