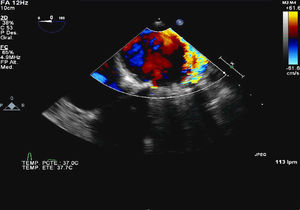
A 62-year-old woman with a history of rheumatic mitral valve disease and commissurotomy closed in 1969, implantation of a Shiley biological mitral prosthesis in 1978, and its replacement in 1998 by a No. 25 Bjork mechanical prosthesis, showed symptoms consistent with congestive heart disease and analytical findings indicating hemolytic anemia. Transesophageal echocardiography detected 2 mitral periprosthetic leaks, a severe one at P2 and a mild one at P3 (Figure 1).
Figure 1.
A decision was made to close the P2 leak, but because the patient had undergone 3 previous heart surgeries, a minimally invasive approach by transapical route was chosen, this being the simplest technique.
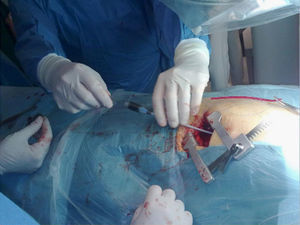
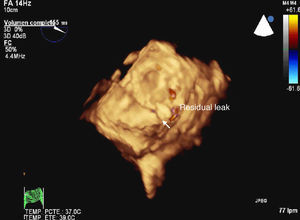
The procedure was carried out in the operating room with the patient under general anesthesia. A left minithoracotomy was performed (Figure 2) with apical access, and an Amplatzer device was implanted over the P2 defect under radioscopic and transesophageal echocardiographic guidance (Figure 3); the outcome was satisfactory.
Figure 2.
Figure 3.
At hospital discharge 5 days later, the patient was asymptomatic and laboratory analyses showed no hemolysis. At 10 days, a transesophageal echocardiographic follow-up study showed that the procedure had been effective, with only a minimal residual leak.
Periprosthetic leaks are a relatively common finding (2% to 3% of mitral prosthesis implantations). When they are severe, they tend to produce symptoms of congestive heart failure or hemolytic anemia.
Percutaneous closure is usually contemplated in patients with a history of several previous heart surgeries or multiple comorbid conditions. An anterograde (transapical) or retrograde (trans-septal) approach can be performed, with success rates of 60% to 90%.
Corresponding author: jdavidrodrigo@gmail.com