Mitral regurgitation (MR) is one of the most prevalent valvular heart diseases. However, only a small proportion of patients are referred for its surgical correction. This type of undertreatment has a multifactorial basis (ie, underestimation of disease risk, high surgical risk, or uncertain benefit in functional MR). Transcatheter mitral valve replacement has emerged as a therapeutic option for patients with severe MR and anatomies unsuited to current percutaneous treatments, such as transcatheter edge-to-edge MR repair (the treatment of choice in MR in patients with suitable valve morphology: valve area, posterior leaflet length, coaptation length, etc) and transapical neochord implantation (reserved for prolapsed MR).1
The Tendyne bioprosthetic mitral valve system (Abbott Vascular, USA) is a self-expanding fully repositionable and retrievable transcatheter mitral valve replacement used in transapical implantation procedures. It has a D-shaped self-expanding nitinol outer frame and a circular self-expanding nitinol inner frame supporting a porcine pericardial tri-leaflet valve. The prosthesis is anchored to the apex with a braided fiber tether and a Teflon apical pad on the epicardium. It is available in 13 sizes with 2 profiles: standard (SP) or low (LP) for smaller native annulus dimensions.
The assessment of patient suitability and careful planning are essential.2 Selection of the prosthesis model and size is based on 3-dimensional (3D) modelling of the native mitral valve and left ventricular outflow tract (LVOT) created by valve replacement. Preoperative study with cardiac computed tomography and transesophageal echocardiogram (TEE) is needed to accurately assess valve geometry and its relationship to ventricular structures, determine the apex approach zone, and choose the valve size to provide a proper fit for paravalvular sealing, device stability, and LVOT area. Recommendations suggest a minimum averaged neo-LVOT area > 250 mm2 (> 325 mm2 in LP valves), ≥ 5mm of tether within the left ventricle, and absence of systolic anterior mitral valve motion.
We present the first 2 implantations in Spain performed in a single center in 2 patients with severe symptomatic MR, high surgical risk, and no alternative transcatheter transfemoral treatment. The patients gave written consent for the scientific dissemination of their cases.
The procedures were performed in the operating room by 2 cardiac surgeons and 1 interventional cardiologist, under general anesthesia, 3D TEE, and fluoroscopic guidance and aortic gradient monitoring to detect LVOT obstruction during implantation.
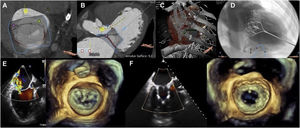
The first intervention was performed in a 79-year-old man with ischemic cardiomyopathy, a left ventricular ejection fraction of 42%, chronic renal failure of 8.6% according to the Society of Thoracic Surgeons (STS) classification, and functional MR due to posterior leaflet retraction with anatomical characteristics unsuited to MitraClip (leaflet length <4mm). A 33-mm SP Tendyne prosthesis was implanted without incident. The correct position of the prosthesis, absence of MR, LVOT obstruction (Vmax=1.4 m/s), stenosis (mean gradient=2mmHg), or paravalvular leaks were verified (figure 1). He was discharged on the fifth day without complications.
A-D: planning using computed tomography. A and B: measuring the annulus, implant simulation, and calculating the new left ventricular outflow tract (295 mm2). C: transapical access. D: fluoroscopic angulation. E and F: pre- and postprocedural mitral regurgitation visualized by 3D transesophageal echocardiography.
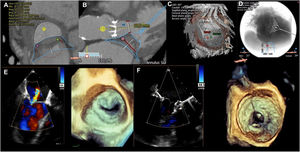
The second intervention was performed in a 65-year-old woman with rheumatic valve disease and a mechanical aortic valve replacement that had been performed 25 years earlier. She had mild stenosis and severe MR with moderate calcification, a left ventricular ejection fraction of 37%, porcelain aorta, chronic lung disease, and severe tricuspid regurgitation with right ventricular dysfunction (STS=28.2%). The use of MitraClip had already been attempted in another hospital, but failed due to a significant increase in the gradient. A 29-mm LP Tendyne prosthesis was successfully implanted with no leaks, stenosis (mean gradient=3mmHg), or LVOT obstruction (Vmax=1 m/s) (figure 2). The patient required a longer admission for predominantly right-sided heart failure and was discharged after 18 days after adjustment of treatment.
A-D: planning using computed tomography. A and B: measuring the annulus, implant simulation, and calculating the new left ventricular outflow tract (480 mm2). C: transapical access. D: fluoroscopic angulation. E and F: pre- and postprocedural mitral regurgitation visualized by 3D transesophageal echocardiography.
The high prevalence of MR and the limitations of surgery in high-risk patients are the main incentive for the development of new transcatheter technologies. Although transcatheter mitral valve replacement using transapical access may prove to be short-lived given the imminent advent of transfemoral prostheses, the Tendyne device increases the therapeutic options and the experience gained with this technique will strongly contribute to its development.
The early efficacy and safety of the Tendyne system has been demonstrated in selected patients.3 An initial experience with 100 patients showed a technical success rate of 96% and no intraoperative mortality. Mortality and stroke rates at 30 days were 6% and 2% respectively, with no MR in 98% of patients.4 Although the device is in the early stages of postmarketing clinical investigation, the high rate of successful implementations, low rate of procedural complications, and device stability make it a treatment of increasing relevance.
The success of the procedure depends on correct patient selection, appropriate imaging techniques, and the joint work of interventional cardiologists, imaging experts, and cardiac surgeons trained in transcatheter procedures for structural heart disease. Although the immediate outcome of these 2 cases has been highly favorable, long-term safety and efficacy results must be obtained to gradually expand its use.
FUNDINGNone declared.
AUTHORS’ CONTRIBUTIONSP. Mahía Casado, J. Cobiella Carnicer, L. Nombela-Franco, and M. Carnero Alcázar contributed to the writing of the article; P. Jiménez Quevedo, L. Nombela-Franco, P. Mahía Casado, and M. Carnero Alcázar contributed to data collection; J. Cobiella Carnicer, L. Nombela-Franco, P. Jiménez Quevedo, M. Carnero Alcázar, and L.C. Maroto Castellanos contributed to the supervision of the article; all authors validated the article.
CONFLICTS OF INTERESTL. Nombela-Franco and J. Cobiella Carnicer work as proctors for Abbot. The other authors declare no conflicts of interest.