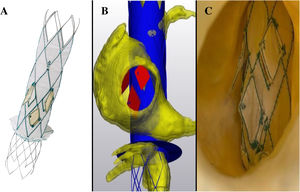
An 82-year-old woman with a history of iatrogenic aortic dissection repaired with an aortic valved conduit (Labcor-23), and severe mitral regurgitation repaired with MitraClip (Abbott, United States) was admitted due to congestive heart failure. Transesophageal echocardiography showed torrential tricuspid regurgitation with suboptimal anatomy for percutaneous edge-to-edge repair (coaptation point displaced towards the apex, pseudoprolapse of the anterior leaflet, and a cleft on the posterior leaflet). The right ventricle showed mild dysfunction (tricuspid annular plane systolic excursion was 14mm and shortening fraction was 43%). Computed tomography showed a short distance from the suprahepatic veins to the right atrium (3.8mm), indicating that the patient was not a good candidate for other heterotopic valves. The decision was made to implant Trillium (Innoventric, Israel) (figure 1), under compassionate use.
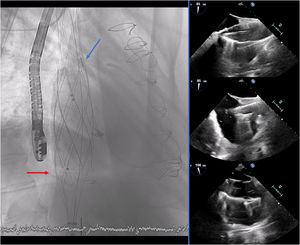
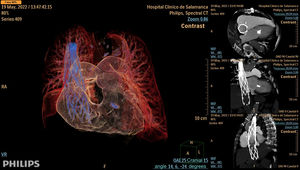
The 24-Fr delivery system was advanced over an extra-stiff wire (Lunderquist, Cook-Medical, United States) and the prosthesis was deployed from the superior to inferior cava veins. The 3 valves of the prosthesis were guided toward the tricuspid valve using radiopaque markers. The prosthesis was successfully implanted without complications (figure 2 and figure 3) (), with a significant decrease in v-wave pressure in both cava veins.
Trillium is a new heterotopic bicaval prosthesis composed of a bare metal stent with radiopaque markers (blue-arrow) for precise deployment under fluoroscopy guidance. The main differences with other heterotopic devices are: it has 3 lateral valves (each of them can be potentially crossed, ie, with a pacemaker lead without interference on the function of the other 2) and a sealing skirt (red-arrow) that prevents any potential leak without obstruction of the suprahepatic veins (one of the main anatomical limitation of the other heterotopic valves). To the best of our knowledge, this is the first case reported of the use of this new prosthesis.
INFORMED CONSENTThe patient's consent was obtained for this publication.
FUNDINGThis paper was not supported by any funding or other sources.
AUTHORS’ CONTRIBUTIONSS. López-Tejero and J. Martín-Moreiras reviewed the literatura on the topic and drafted the manuscript. I. Cruz-González led the research and supervised manuscript drafting and revised the final versión.
CONFLICTS OF INTERESTThe authors have no conflict of interest in relation to this paper.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.12.008