The treatment of coronary chronic total occlusions (CTO) remains a challenge for the interventional cardiologist. Failure of balloon angioplasty is the second more common cause of an unsuccessful procedure. We describe our experience with the use of the new Tornus® catheter (Asahi Intecc, Aichi, Japan) designed specifically for the treatment of “nondilatable” CTO. Between November 2008 and March 2010, 17 patients (age 62 years, 88% men, 82% dyslipidemia, 52% hypertension, 29% diabetes) were treated in whom balloon dilatation had failed after crossing the lesion with the guide. The use of Tornus® catheter was successful without complications in 15. All patients underwent clinical follow-up (median, 573 days) with no documented major adverse events. The use of the Tornus® catheter is safe and feasible in those patients with CTO lesions in whom balloon angioplasty has been unsuccessful.
Keywords
Almost 30 years after the introduction of angioplasty, the recanalization of coronary chronic total occlusion (CTO) has been defined as the “final frontier” in interventional cardiology.1 The difficulty in its treatment is reflected in a lower success rate compared to other lesions (70% vs 98%).2 The benefits of recanalization include symptom relief, improvement in left systolic ventricular function and better long-term survival.3 However, its percutaneous treatment only represents 10%-15% of all angioplasties4 and its presence remains the most frequent cause of failure in revascularization procedures.1 According to data provided by the Sección de Hemodinámica y Cardiología Intervencionista de la Sociedad Española de Cardiología (Cardiac Catheterization and Interventional Cardiology Section of the Spanish Society of Cardiology) registry of 2008, only 2.8% of the 61 810 angioplasties performed involved CTO.5
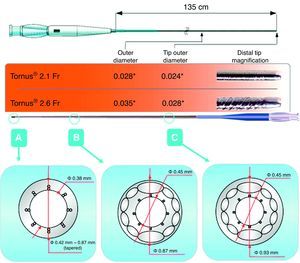
During the last decade, new techniques and instruments have been developed to overcome these problems.6, 7, 8 Among the new instruments is the Tornus® catheter (Asahi Intecc, Aichi, Japan). This catheter can accept 0.014-inch coaxial guides, and is made up of 8 braided steel wires along the longitudinal axis providing it with its penetrative capacity and flexibility when rotated.
The aim of this study was assess the feasibility and usefulness of the Tornus® catheter in CTO in which revascularization had not been successful due to the inability to achieve dilatation using any of the balloon catheters available.
Methods Selection CriteriaBetween February 2006 and March 2010, 247 patients with 268 CTO were treated. Between November 2008 (when the device became available) and March 2010, the Tornus® catheter was used in 17 patients with CTO in whom balloon (diameters between 1 mm-1.25mm and 1.5mm) dilatation had failed after crossing the lesion with the guide. These patients formed the study population. No exclusion criteria were applied.
DefinitionsAngiographic success was defined as obtaining vessel patency with a residual stenosis of <30% and TIMI III flow. Major adverse events included: all-cause death, myocardial infarction and the need for target-vessel revascularization.
Use of the Tornus® DeviceOnce the guide has crossed the CTO, a 0.014-inch guide extension is added, into which a 2.1 or 2.6 Fr Tornus® catheter (Figure 1, Figure 2) is inserted. As the guide acts as a mandrel for the catheter, the guide must be held steady while the catheter is rotated. This is advanced by anticlockwise rotation and withdrawn by clockwise rotation. No more than 20 rotations in the same direction should be performed in order to prevent the risk of guide fracture, which could lead to the system blocking the artery.
Figure 1. Structure of the Tornus® catheter.
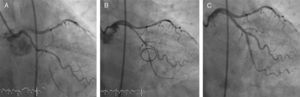
Figure 2. Usefulness of the Tornus® catheter. A, baseline angiography; occlusion at the level of the ostium of the second marginal branch at the bifurcation with the first marginal branch, with marked angulation in relation to the proximal circumflex artery. B, guide in the distal bed of the marginal branch; it was not possible to cross the bifurcation with any balloon; control angiography after advancing the guide in the distal vessel bed having used the Tornus® catheter. C, final outcome.
Balloon Angioplasty and Stent ImplantationSuccessful deployment of the device was followed by dilatation using balloon catheters; the initial diameters were 1.5 mm-2mm, and were subsequently adapted to the diameter of the vessel. Stents were implanted in all the patients in whom dilatation was successful.
MedicationAll the patients received 100mg aspirin before the procedure and indefinitely thereafter. A loading dose of 300mg clopidogrel was administered to those who were not already receiving this drug, followed by 75mg daily for 12 months in patients implanted with a drug-eluting stent.
Clinical follow-up was conducted by telephone or at the clinic.
ResultsThe mean age of the study population was 62 (58-77) years, of whom 88% were men. In total, 82% had a history of dyslipidemia; 52% had hypertension and 29% had diabetes mellitus (Table 1). The procedure was reattempted in 5 patients in whom the revascularization procedure had been performed before the device became available. The remaining patients came from a more recent period and in most of them the catheter was used in a first revascularization procedure after dilatation had failed using the low-profile balloons available at that time. The characteristics of the procedure are described in Table 2. Revascularization was successful using the Tornus® device in 15 of the 17 cases (88%). No technical problems occurred (side branch embolization, vessel thrombosis or distal embolization, spasm, rupture or device entrapment) or ischemic or arrhythmic events. The only complication of note was proximal dissection of the vessel secondary to advancing the device through a tortuous artery using a catheter guide that provided insufficient support. This problem was overcome by the implantation of an additional stent. In 2 cases it was not possible to cross the occlusion even partially with the catheter, leading to revascularization failure; both cases involved very distal and intensely calcified occlusions. All patients underwent clinical follow-up (median, 573 days) and no major adverse events were observed.
Table 1. Clinical Characteristics.
| Patient | Age (years) | Sex | AHT | DLP | Smoking | DM |
| 1 | 58 | M | Yes | Yes | Yes | No |
| 2 | 70 | W | Yes | No | Yes | No |
| 3 | 60 | M | No | Yes | Yes | No |
| 4 | 73 | M | Yes | Yes | Yes | Yes |
| 5 | 60 | M | No | Yes | No | No |
| 6 | 55 | M | Yes | Yes | No | Yes |
| 7 | 59 | M | No | Yes | Yes | No |
| 8 | 51 | M | No | Yes | Yes | No |
| 9 | 58 | M | No | Yes | Yes | No |
| 10 | 77 | W | Yes | Yes | No | Yes |
| 11 | 49 | M | Yes | Yes | No | No |
| 12 | 56 | M | Yes | Yes | No | Yes |
| 13 | 69 | M | Yes | Yes | No | No |
| 14 | 62 | M | No | Yes | Yes | Yes |
| 15 | 68 | M | No | No | Yes | No |
| 16 | 68 | M | No | No | No | No |
| 17 | 58 | M | Yes | Yes | Yes | No |
AHT, arterial hypertension; DLP, dyslipidemia; DM, diabetes mellitus; M, man; W, woman.
Table 2. Characteristics of the Procedure.
| Patient | First procedure (years) | Vessel | Tornus® size | Attempts | Final guide-wire | Fluoroscopy time (min) | Duration of the procedure (min) | Contrast agent (mL) | Complications | Success |
| 1 | 2007 | RC | 2.1 | 3 | Miracle 3 | 121 | 315 | 1119 | No | Yes |
| 2 | 2008 | Ostium, first OM | 2.1 | 2 | Miracle 3 | 50 | 155 | 356 | PD | Yes |
| 3 | 2008 | RC | 2.1 | 2 | Miracle 3 | 72 | 131 | 557 | No | Yes |
| 4 | 2008 | RC | 2.1 | 3 | Fielder | 84 | 182 | 432 | No | Yes |
| 5 | 2009 | RC | 2.1 | 1 | Miracle 6 | 59 | 120 | 235 | No | Yes |
| 6 | 2009 | CX | 2.1 | 1 | Pilot 200 | 35 | 75 | 171 | No | Yes |
| 7 | 2009 | CX | 2.1 | 1 | Miracle 3 | 52 | 140 | 456 | No | Yes |
| 8 | 2009 | AD | 2.1 | 1 | Miracle 3 | 39 | 120 | 142 | No | Yes |
| 9 | 2009 | RC | 2.1 | 2 | Miracle 3 | 93 | 265 | 406 | No | Yes |
| 10 | 2009 | AD | 2.1 | 1 | Fielder | 46 | 110 | 200 | No | Yes |
| 11 | 2009 | RC | 2.1 | 1 | Fielder | 11 | 143 | 207 | No | Yes |
| 12 | 2009 | RC | 2.1 | 1 | Miracle 6 | 33 | 70 | 217 | No | Yes |
| 13 | 2009 | AD | 2.1 | 1 | Miracle 3 | 41 | 120 | 300 | No | Yes |
| 14 | 2010 | RC | 2.1 | 1 | Miracle 3 | ? | 152 | 400 | No | Yes |
| 15 | 2010 | RC | 2.1 | 1 | Miracle 3 | ? | 109 | 140 | No | No |
| 16 | 2010 | RC | 2.6 | 1 | Confidence | ? | 96 | 90 | No | Yes |
| 17 | 2007 | RC | 2.1 | 3 | Fielder XT | 120 | 352 | 532 | No | No |
AD, anterior descending; CX, circumflex; OM, obtuse marginal; PD, proximal dissection; RC, right coronary.
Despite the technical advances of the last 15 years, the success rates of CTO recanalization range between 50% and 85%, depending on the experience of the operator and lesion complexity.9 In the literature, the most frequent cause of failure is the inability to cross the occluded area with the angioplasty guide (80%-90%), followed by failure to cross the occlusion with a balloon (2-15%) or dilatation failure (2%-5%).10 In our series, the Tornus® catheter was used with a high success rate (88%) in a subgroup of patients who were particularly difficult to manage — those with CTO in whom dilatation with a low-profile balloon had failed — thus reducing the overall failure rate.
The Tornus® catheter has 3 functions. Due to the improved angioplasty system support as compared to a microcatheter or a coaxial balloon, it increases the capacity of the guide to penetrate the occlusion, permits safe guide exchange without damaging the proximal vessel, and creates a channel in the body of the occlusion, since it acts like a corkscrew. The creation of this channel reduces the risk of dissection that could occur when inflating a balloon and facilitates the dilatation of those lesions through which a balloon cannot be crossed. In Spain, 2 sizes are available: a more flexible 2.1Fr device, recommended in tortuous lesions and in side branches with a very angulated origin (Figure 1); and a 2.6Fr device, with greater penetrative power and support whose use is recommended in straight vessels and lesions of greater hardness (Figure 2). Due to its size and structure, the Tornus® catheter is not suitable in procedures using the retrograde approach.
The first description of the catheter and its clinical use was provided by Tsuchikane et al.,11 who reported a success rate of 100% in 14 patients — 10 of whom had CTO — with severe lesions and calcified vessels that impeded the advance of balloons or microcatheters.
It is common to offer a second opportunity to patients in whom attempted revascularization has failed. Thus, the use of this type of catheter, following dilatation failure using conventional low-profile balloon catheters, can avoid repeat procedures, reduce the administration of contrast agents and radiation energy, and decrease costs.
One of the greatest limitations of this device is its reduced capacity to penetrate lesions with intense superficial calcification. In one unsuccessful case in our series, a coronary artery CT scan in the sagittal view showed 100% calcification of the circumference of the target vessel in the occlusion area. Cases of this type can only be treated by using an ablation technique (Rotablator®).
This catheter cannot replace conventional microcatheters in long procedures, since it carries a greater risk of thrombosis, given that the metal is in direct contact with the blood and flushing cannot be performed.
To date, the published studies describe personal experiences of using the device in “non-dilatable” patients, and no randomized prospective studies have compared the catheter with other techniques.
Consistent with the cited studies, our results show that managing the catheter, although not free of risk, is not complex providing the manufacturer's instructions are followed, but it requires a catheter guide that provides good support and its advance can be difficult in very tortuous arteries.
As described previously,12 once the use of the catheter has become familiar, we can expand its indication to patients with very tortuous, moderately calcified vessels in whom it has not been possible to advance any type of balloon catheter.
LimitationsAs this was an observational study that describes our group's experience with a small series, the results cannot be generalized.
Conflicts of interestNone declared.
Received 19 October 2010
Accepted 14 January 2011
Corresponding author: Unidad de Hemodinámica, Servicio de Cardiología, Hospital Clínic, Villarroel 170, 08036 Barcelona, Spain. 27700vmy@comb.cat