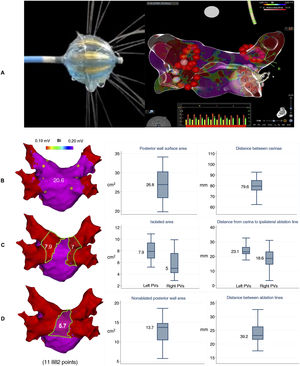
Pulmonary vein isolation (PVI) is the cornerstone of invasive atrial fibrillation treatment. Robust evidence, however, is lacking on the influence of lesion size after isolation of pulmonary veins in the posterior left atrial wall and fibrillation recurrence. Several studies have analyzed lesion extent following PVI performed with a range of techniques, including cryoballoon and laser balloon ablation.1,2 The Heliostar radiofrequency balloon catheter system (Biosense Webster Inc, USA) can achieve single-shot PVI and integrates with the CARTO 3 navigation system for full visualization (Biosense Webster, Israel). The Heliostar combines 10 longitudinal electrodes for tailored energy delivery and a module for real-time visualization of temperature and local impedance (figure 1A). The extent and characteristics of lesions following PVI with radiofrequency balloon ablation are unknown.
A: left, the Heliostar radiofrequency balloon catheter during maximum irrigation; right, voltage map from the CARTO 3 electroanatomic mapping system showing posterior view of the left atrium during delivery of radiofrequency energy to the right inferior pulmonary vein (note the ablation lines encircling the other veins); bottom, ablation panel showing real-time impedance, temperature and voltage data for each electrode. B: left, preablation voltage map (posterior view of left atrium) for 1 of the patients studied; the yellow dots show the anatomically and electrically defined pulmonary vein ostium and enclose the entire surface area of the posterior wall (20.6cm2 in the example shown); right, box plots showing posterior wall surface area and distance between carinae for the total population (median, interquartile range, minimum, and maximum). C: left, postablation voltage map, with green lines showing the isolated posterior wall area at the antra of the left and right veins; right, box plots showing posterior wall surface area and distance from carina to the ipsilateral ablation line for total population. D: left, postablation voltage map, with green lines showing nonablated posterior wall area; right, box plots showing nonablated posterior wall area and distance between ablation lines at the level of the carinae. The maps shown included 11 882 points. Bi, bipolar; PVs, pulmonary veins.
The aims of this study were to characterize lesions produced by PVI with the Heliostar radiofrequency balloon catheter and to analyze potential predictors of lesion size and quality. This observational, nonrandomized, noncomparative study was approved by the local health care ethics committee (Ref. CI 22/548-P_NoEC). Signed informed consent was obtained from all patients.
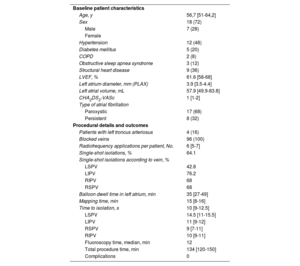
We studied 25 consecutive patients with a history of paroxysmal or persistent atrial fibrillation of less than 1 year who underwent PVI with the Heliostar radiofrequency balloon catheter using a methodology described elsewhere.3 Baseline patient characteristics, procedural details, and outcomes are summarized in table 1. The CARTO 3 navigation system (Biosense Webster) was used to generate a high-density electroanatomic map of the left atrium before and after ablation. The median [interquartile range (IQR)] number of points acquired was 3219 [2294-4633 points].
Baseline patient characteristics and procedural details and outcomes
| Baseline patient characteristics | |
| Age, y | 56,7 [51-64,2] |
| Sex | 18 (72) |
| Male | 7 (28) |
| Female | |
| Hypertension | 12 (48) |
| Diabetes mellitus | 5 (20) |
| COPD | 2 (8) |
| Obstructive sleep apnea syndrome | 3 (12) |
| Structural heart disease | 9 (36) |
| LVEF, % | 61.6 [58-68] |
| Left atrium diameter, mm (PLAX) | 3.9 [3.5-4.4] |
| Left atrial volume, mL | 57.9 [49.9-83.8] |
| CHA2DS2-VASc | 1 [1-2] |
| Type of atrial fibrillation | |
| Paroxystic | 17 (68) |
| Persistent | 8 (32) |
| Procedural details and outcomes | |
| Patients with left troncus arteriosus | 4 (16) |
| Blocked veins | 96 (100) |
| Radiofrequency applications per patient, No. | 6 [5-7] |
| Single-shot isolations, % | 64.1 |
| Single-shot isolations according to vein, % | |
| LSPV | 42.8 |
| LIPV | 76.2 |
| RIPV | 68 |
| RSPV | 68 |
| Balloon dwell time in left atrium, min | 35 [27-49] |
| Mapping time, min | 15 [8-16] |
| Time to isolation, s | 10 [9-12.5] |
| LSPV | 14.5 [11-15.5] |
| LIPV | 11 [9-12] |
| RSPV | 9 [7-11] |
| RIPV | 10 [9-11] |
| Fluoroscopy time, median, min | 12 |
| Total procedure time, min | 134 [120-150] |
| Complications | 0 |
CHA2DS2-VASc, acronym for congestive heart failure; hypertension, age ≥ 75 y (double score), diabetes mellitus, stroke (double score) vascular disease, age 65-74 years and sex (female); COPD, chronic obstructive pulmonary disease; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; LVEF, left ventricular ejection fraction; PLAX, parasternal long axis; OSA, obstructive sleep apnea syndrome; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Unless otherwise indicated, values are expressed as No. (%) or median [interquartile range].
The electroanatomic maps were processed using a previously described method.1,2 The ostium of each pulmonary vein was anatomically and electrically defined prior to ablation.4 The generated maps were used to define the isolation areas on the left and right posterior walls between the respective ostia and ipsilateral ablation lines. Voltage areas of >0.2mV were used to identify healthy tissue (figure 1B-D). The isolated surface area accounted for a median of 52.2% [IQR, 45%-56.6%] of the total posterior wall.
We analyzed atrial volume, distance between carinae, and the angle of the pulmonary veins with respect to the plane of the posterior wall. None of these variables was significantly associated with the isolated atrial segments.
Single-shot PVI was achieved in 64.1% of cases. Significant predictors of single-shot PVI in the univariate analysis using multilevel or mixed effects logistic regression were impedance drop (odds ratio [OR), 0.1 per drop of 1Ω; 95%CI, 0.002-0.2; P=.045) and temperature rise (OR, 0.17 per 1-C° rise; 95%CI, 0.017-0.33; P=.03). Minimum voltage recorded by the balloon electrodes after PVI showed a tendency to predict single-shot isolation, but the association did not reach statistical significance (OR, –0.9 for 1-mV increase; 95%CI, –2 to 0.3; P=.1). The model that best predicted single-shot isolation included atrial volume, mean impedance drop, maximum impedance drop, and mean temperature rise (sensitivity, 72%; specificity, 68%; area under the curve, 0.75). There were no atrial fibrillation recurrences in 92% of patients over a median follow-up of 12 months (Holter monitoring and electrocardiograms at 3, 6, 9, and 12 months). A blanking period of 3 months was used, and antiarrhythmic treatment was maintained in all cases until the first visit.
PVI with a radiofrequency balloon catheter produces very antral lesions and an isolation area occupying 52% of the posterior wall. Our study shows that radiofrequency balloon catheter ablation results in more extensive lesions and posterior wall isolation than cryoballoon ablation.1 Similar or larger extents have been reported for electroporation. 2,5 The influence on PVI with radiofrequency balloon ablation on atrial fibrillation recurrence remains unclear. Both impedance drop and temperature rise predicted single-shot isolation, confirming recent findings.6
FUNDINGThis study received no funding.
ETHICAL CONSIDERATIONSThis study was approved by the local health care ethics committee (Ref. CI 22/548-P_NoEC). Informed consent was obtained from all patients for the conduct and publication of this study. Sex and gender were reported in accordance with the Spanish Sex and Gender Equity in Research (SAGER) guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEArtificial intelligence was not used in this study.
AUTHORS’ CONTRIBUTIONSE. Martínez Gómez: protocol design, data collection, statistical analysis, and manuscript drafting. Salgado protocol design, statistical analysis, and manuscript revision; D. Calvo Cuervo: manuscript revision. C. Sánchez Vallejo: data collection. D. Filgueiras-Rama: manuscript revision. N. Pérez-Castellano: protocol design, statistical analysis, and manuscript review.
CONFLICTS OF INTERESTD. Filgueiras-Rama is associate editor of Revista Española de Cardiología. The journal's editorial procedure was followed to guarantee the impartial handling of the manuscript.
The other authors do not report any conflicts of interest.