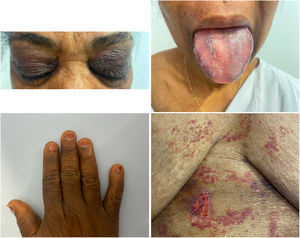
A 60-year-old woman from Equatorial Guinea consulted for shortness of breath during the previous month and swelling of the lower extremities. On physical examination, blood pressure was within the normal range, with slight tachycardia. The patient exhibited signs of biventricular congestion, hepatomegaly, periorbital erythema, macroglossia, nail dystrophy, and erosive skin lesions below the sternum (figure 1). Signed consent was obtained for acquisition of the photographs.
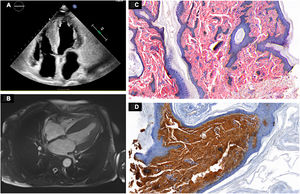
Clectrocardiogram showed low voltage, and echocardioscopy depicted biventricular hypertrophy, speckled myocardium, and a restrictive diastolic pattern. Based on these findings, the patient was hospitalized for further study. On laboratory testing, NT-proBNP value was 1261 pg/mL and protein electrophoresis depicted a monoclonal band in the beta region and increased lambda light chains (80.47mg/dL). The 24-hour urine study showed proteinuria within the nonnephrotic range (1.93g/L). Echocardiography findings included biventricular hypertrophy, moderately reduced systolic function with restrictive features, and significant biatrial enlargement (figure 2A). Magnetic resonance imaging (figure 2B) depicted extensive diffuse thickening of the left ventricle, left ventricular ejection fraction at 45%, diffuse fibrosis, and significantly increased extracellular volume (53%), consistent with advanced cardiac amyloidosis. Skin biopsy specimens tested positive on Congo Red staining and the immunohistochemical profile was positive for lambda light chains (figures 2C,D), thereby confirming the diagnosis of systemic primary amyloidosis. The patient was started on chemotherapy.
This case study documents both the cardiac and extracardiac manifestations of primary amyloidosis, synthesizing the clinical diagnosis and complementary test findings in this condition.
FUNDINGNone.
ETHICAL CONSIDERATIONSInformed consent was obtained for this case report: the patient understood the importance of performing the tests and obtaining photographs, viewed the photographs, was aware of the purpose of publishing the case, and agreed to and signed the consent forms. The pertinent rules and guidelines for conducting the study were followed. No artificial intelligence was used. The sex and gender considerations outlined in the SAGER guidelines are not applicable in this case.
AUTHORS’ CONTRIBUTIONSAll authors contributed to conducting the tests and writing the manuscript.
CONFLICTS OF INTERESTNone.