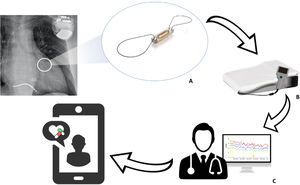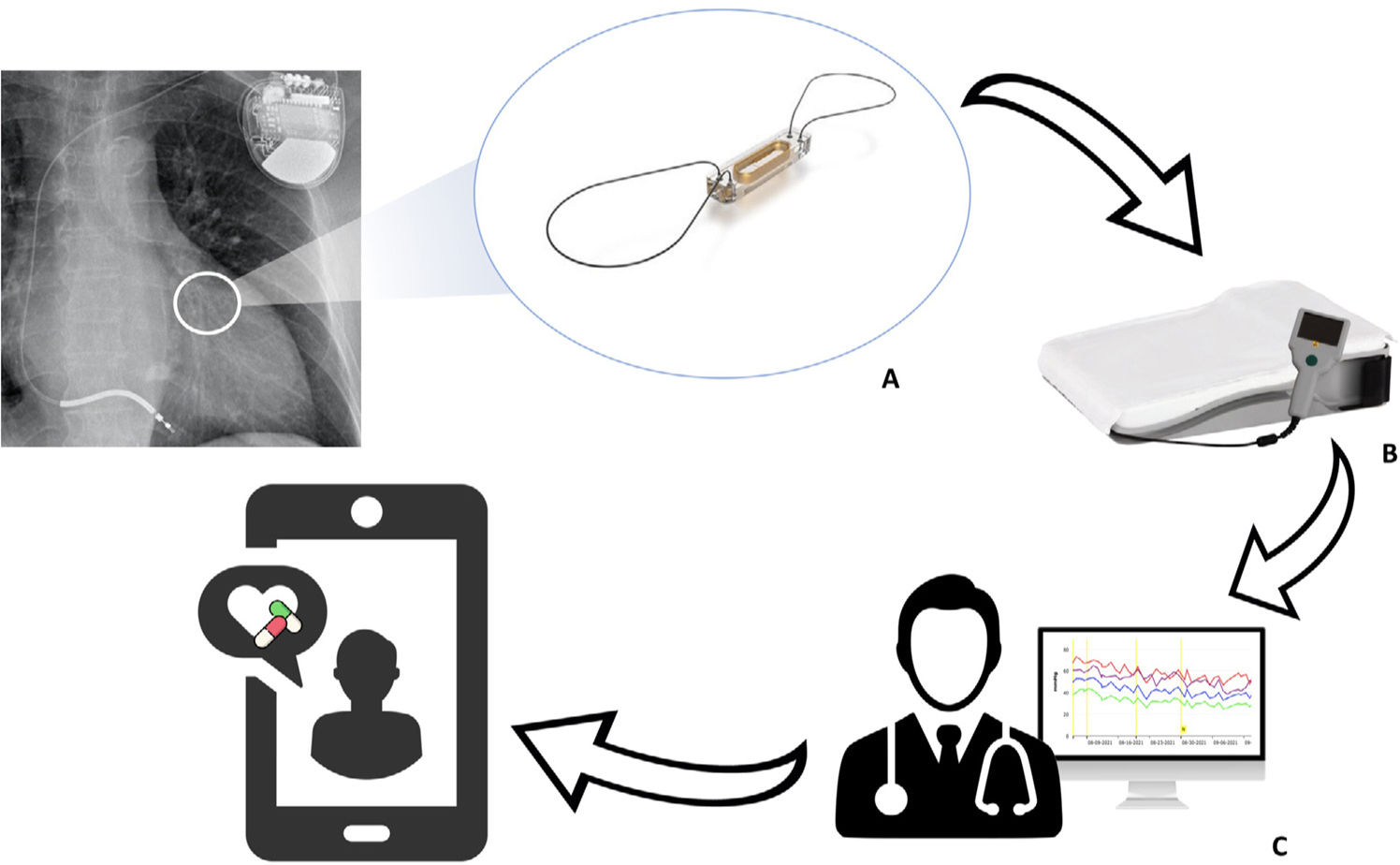
Pulmonary hypertension (PHT) is a common finding in patients with heart failure (HF) and has prognostic relevance.1 Increasing pulmonary congestion resulting from HF is accompanied by adaptive changes in the pulmonary circulation (remodeling of the vasculature and extracellular matrix) that lead to increased pulmonary vascular resistance (PVR) and combined precapillary and postcapillary PHT, which is frequent in patients with advanced HF.2 Medical treatment guided by remote pulmonary arterial pressure monitoring (RPAPM) based on the wireless CardioMEMS device (Abbott, United States) implanted in the pulmonary artery reduces HF hospitalizations3,4 and significantly decreases PHT.5 CardioMEMS comprises the following components: a sensor with a pressure-sensitive capacitor that is placed inside a branch of the pulmonary artery using right cardiac catheterization (RCC); an electronic system that receives the pressure signal and transmits it on activation by the patient; and a software application that enables interpretation of the signal (figure 1). Readings taken via RCC in the implant help set hemodynamic targets to guide treatment.
Evaluation of PHT is a key element in pretransplant work-up. Irreversible PHT, defined as systolic pulmonary artery pressure >50mmHg and PVR >3 Wood units or a transpulmonary gradient >15mmHg, is considered a contraindication for isolated heart transplant. This assessment is performed using RCC and, in the case of PHT, requires the patient to initiate drug therapy to reverse the increase in PVR (diuretics, inotropic agents, or pulmonary vasodilators such as prostaglandins, phosphodiesterase 5 [PDE5] inhibitors, and endothelin receptor antagonists) or be implanted with a left ventricular assist device.6 The hemodynamic status of patients on the transplant waiting list should be re-evaluated periodically using RCC (generally every 3-6 months). Nevertheless, given the high frequency of decompensation in these patients and the unforeseeable nature of transplant scheduling, such a strategy may be insufficient for predicting the grade of PHT at transplant, with an increase in the posttransplant risk of right-sided HF. Studies evaluating the effectiveness of RPAPM devices show that patients in New York Heart Association functional class IV are underrepresented and that transplant candidates are excluded.3 However, RPAPM could prove useful in these patients, since it enables closer monitoring and treatment adjustment. This report aims to review preliminary experience in the use of RPAPM to guide the treatment of patients on the heart transplant waiting list.
The CardioMEMS RPAPM program at our center was started in September 2019. The system was implemented in 5 waiting list patients between November 2020 and October 2023. Pulmonary arterial pressure (PAP) readings were evaluated twice weekly by a physician from the HF unit. If the hemodynamic targets changed significantly, eg, increases of 3 to 5mmHg in diastolic pulmonary arterial pressure (PAPd) for 5 days or 5 mmHg in 1 day, the patient was contacted to adjust treatment or given an appointment for intravenous treatment or admission to hospital. If PAP did not improve, RCC was repeated to re-evaluate PVR and the patient's suitability for transplant. In addition, all patients were evaluated in person at least every 4 to 6 weeks.
Patient characteristics are summarized in table 1. The patients selected for RPAPM were those who required specific medical treatment to revert PVR (inotropic drugs and PDE5 inhibitors) in the RCC during the pretransplant work-up or those with significantly elevated filling pressure in the follow-up RCCs while on the waiting list and who required intravenous treatment.
Characteristics of patients on the heart transplant waiting list implanted with a cardiomems device
| Patient | Sex | Age, y | Heart disease | LVEF, % | BMI | Status on waiting list | TPG at implantation, mmHg | DPG at implantation, mmHg | PAPd at implantation, mmHg | Time between implantation and transplant, d | Transplant status | PAPd at transplantation, mmHg | PGF | Alive 30 d after transplant | Posttransplant PAPd, mmHg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 65 | Hypertrophic cardiomyopathy | 50 | 31 | Transplanted | 12 | 5 | 31 | 126 | Urgent | 21 | Yes | Yes | 11 |
| 2 | Male | 67 | Hypertrophic cardiomyopathy | 18 | 25.5 | Transplanted | 10 | 3 | 21 | 76 | Urgent | 20 | No | Yes | — |
| 3 | Male | 59 | Amyloidosis | 61 | 34.7 | Temporarily delisted | 15 | 5 | 21 | — | — | — | — | — | — |
| 4 | Male | 63 | Dilated cardiomyopathy | 17 | 23.6 | Transplanted | 11 | 7 | 36 | 61 | Elective | 31 | No | Yes | 18 |
| 5 | Female | 59 | Valvular | 32 | 31.1 | Temporarily delisted +early relisting | 12 | 3 | 21 | — | — | — | — | — | — |
BMI, body mass index; DPG, diastolic pulmonary gradient; LVEF, left ventricular ejection fraction; PAPd, diastolic pulmonary arterial pressure; PGF, primary graft failure; TPG, transpulmonary gradient.
During a median [interquartile range] follow-up of 75 [70-125] days, 3 patients received a transplant and 2 were temporarily delisted owing to a significant increase in PAP confirmed by RCC at 3 months and at 3 weeks after inclusion. One of the delisted patients was relisted 20 days after hemodynamic optimization with intravenous diuretics and PDE5 inhibitors. Due to the underlying heart disease, none of the patients were considered candidates for a left ventricular assist device.
Two of the transplants were urgent procedures (status 0) with an Impella CP device (Abiomed, United States), and the other was elective. Following the transplant, 1 patient developed right-sided HF requiring implantation of a centrifugal right ventricular assist device immediately after surgery; no complications were detected in the other 2 patients. All 3 patients were discharged.
The median time with RPAPM before transplant was 76 [61-76] days. During this period, treatment was adjusted 4 times for each patient, mainly temporary increases in the oral diuretics dose, although on 4 occasions intravenous treatment was required (diuretics or inotropic drugs [levosimendan]). PAPd values decreased significantly in all patients between implantation and transplant (median, 5 [1-5] mmHg). CardioMEMS readings continued to be taken in 2 patients after transplant, revealing a marked reduction in PAPd (table 1).
The results recorded show the usefulness and safety of RPAPM with implantable devices in selected patients at high risk of worsening PHT while on the transplant waiting list. At the time of their transplant, patients had significantly decreased PHT, and none had experienced implant-related adverse events. Moreover, RPAPM enabled the detection of significant variations in PAP in patients on the waiting list and guided repeat RCC to determine the patient's suitability for transplantation earlier than specified in the protocol. However, various questions remain to be elucidated, such as the most suitable posttransplantation antithrombotic treatment for patients on the waiting list and research lines of interest, including the usefulness of RPAPM in the posttransplant follow-up of these patients.
FUNDINGSeveral of the authors of this article are members of Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, Madrid, which funded the research.
ETHICAL CONSIDERATIONSThe authors state that the content of the present study did not require specific approval by their institution's Clinical Research Ethics Committee. All study patients gave their written informed consent to be included on the heart transplant waiting list and to be implanted with the CardioMEMS remote pulmonary arterial pressure device.
The male-to-female ratio in the present study was 4:1, reflecting the real-world situation of heart transplant in Spain and elsewhere.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEArtificial intelligence was not used in the preparation of the present study.
AUTHORS’ CONTRIBUTIONSAll authors participated in this research, evaluated the results, and agree to their publication.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.